Page 134 - 2023 Taiwan Food and Drug Administration Annual Report
P. 134
2023 Taiwan Food and
Drug Administration
Annual Report
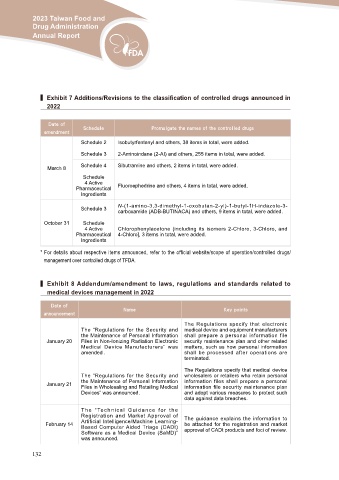
▍ Exhibit 7 Additions/Revisions to the classification of controlled drugs announced in
2022
Date of Schedule Promulgate the names of the controlled drugs
amendment
Schedule 2 Isobutyrfentanyl and others, 38 items in total, were added.
Schedule 3 2-Aminoindane (2-AI) and others, 255 items in total, were added.
March 8 Schedule 4 Sibutramine and others, 2 items in total, were added.
Schedule Fluoroephedrine and others, 4 items in total, were added.
4 Active
Pharmaceutical
Ingredients
Schedule 3 N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-butyl-1H-indazole-3-
carboxamide (ADB-BUTINACA) and others, 9 items in total, were added.
October 31 Schedule
4 Active
Pharmaceutical Chlorophenylacetone (including its isomers 2-Chloro, 3-Chloro, and
Ingredients 4-Chloro], 3 items in total, were added.
* For details about respective items announced, refer to the official website/scope of operation/controlled drugs/
management over controlled drugs of TFDA.
▍ Exhibit 8 Addendum/amendment to laws, regulations and standards related to
medical devices management in 2022
Date of Name Key points
announcement
January 20 The “Regulations for the Security and The Regulations specify that electronic
the Maintenance of Personal Information medical device and equipment manufacturers
Files in Non-Ionizing Radiation Electronic shall prepare a personal information file
Medical Device Manufacturers” was security maintenance plan and other related
amended . matters, such as how personal information
shall be processed after operations are
terminated.
January 21 The “Regulations for the Security and The Regulations specify that medical device
the Maintenance of Personal Information wholesalers or retailers who retain personal
Files in Wholesaling and Retailing Medical information files shall prepare a personal
Devices” was announced. information file security maintenance plan
and adopt various measures to protect such
data against data breaches.
February 14 T h e “ Te c h n i c a l G u i d a n c e f o r t h e The guidance explains the information to
Registration and Market Approval of be attached for the registration and market
Artificial Intelligence/Machine Learning- approval of CADt products and foci of review.
Based Computer Aided Triage (CADt)
Software as a Medical Device (SaMD)”
was announced.
132