Page 130 - 2021 Taiwan Food and Drug Administration Annual Report
P. 130
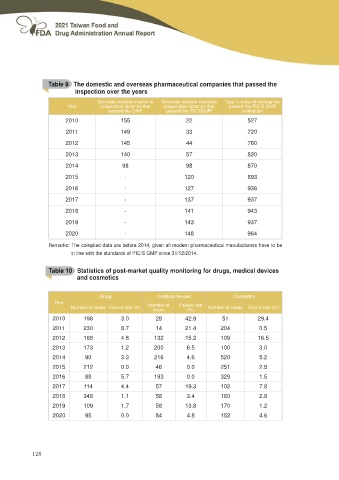
Table 9 The domestic and overseas pharmaceutical companies that passed the
inspection over the years
Year Domestic western medicine Domestic western medicine Total number of companies
preparation factories that preparation factories that passed the PIC/S GMP
passed the GMP passed the PIC/SGMP evaluation
2010 155 22 527
2011 149 33 720
2012 145 44 760
2013 140 57 820
2014 98 98 870
2015 - 120 893
2016 - 127 936
2017 - 137 937
2018 - 141 943
2019 - 143 937
2020 - 148 964
Remarks: The compiled data are before 2014, given all modern pharmaceutical manufacturers have to be
in line with the standards of PIC/S GMP since 31/12/2014.
Table 10 Statistics of post-market quality monitoring for drugs, medical devices
and cosmetics
Drugs Medical devices Cosmetics
Number of cases Failure rate (%)
Year Number of Failure rate
cases (%)
Number of cases Failure rate (%)
2010 198 3.0 28 42.9 51 29.4
2011 230 8.7
2012 168 4.8 14 21.4 204 0.5
2013 173 1.2
2014 90 3.3 132 15.2 109 16.5
2015 212 0.0
2016 88 5.7 200 6.5 100 3.0
2017 114 4.4
2018 348 1.1 216 4.6 520 5.2
2019 109 1.7
2020 95 0.0 46 0.0 251 2.8
193 0.0 329 1.5
57 19.3 102 7.8
58 3.4 180 2.8
58 13.8 170 1.2
84 4.8 152 4.6
128