Page 128 - 2021 Taiwan Food and Drug Administration Annual Report
P. 128
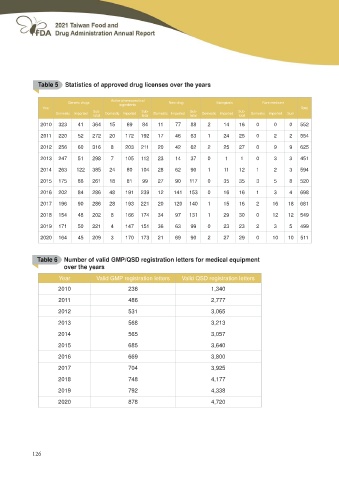
Table 5 Statistics of approved drug licenses over the years
Generic drugs Active pharmaceutical New drug Biologicals Rare medicine
ingredients Domestic Imported Domestic Imported Domestic Imported Sum
Year Total
Domestic Imported Sub- Domestic Imported Sub- Sub- Sub-
total total total total
2010 323 41 364 15 69 84 11 77 88 2 14 16 0 0 0 552
2011 220 52 272 20 172 192 17 46 63 1 24 25 0 2 2 554
2012 256 60 316 8 203 211 20 42 62 2 25 27 0 9 9 625
2013 247 51 298 7 105 112 23 14 37 0 11 0 3 3 451
2014 263 122 385 24 80 104 28 62 90 1 11 12 1 2 3 594
2015 175 86 261 18 81 99 27 90 117 0 35 35 3 5 8 520
2016 202 84 286 48 191 239 12 141 153 0 16 16 1 3 4 698
2017 196 90 286 28 193 221 20 120 140 1 15 16 2 16 18 681
2018 154 48 202 8 166 174 34 97 131 1 29 30 0 12 12 549
2019 171 50 221 4 147 151 36 63 99 0 23 23 2 3 5 499
2020 164 45 209 3 170 173 21 69 90 2 27 29 0 10 10 511
Table 6 Number of valid GMP/QSD registration letters for medical equipment
over the years
Year Valid GMP registration letters Valid QSD registration letters
2010 236 1,340
2011 486 2,777
2012 531 3,065
2013 568 3,213
2014 565 3,057
2015 685 3,640
2016 669 3,800
2017 704 3,925
2018 748 4,177
2019 792 4,338
2020 878 4,720
126