Page 129 - 2021 Taiwan Food and Drug Administration Annual Report
P. 129
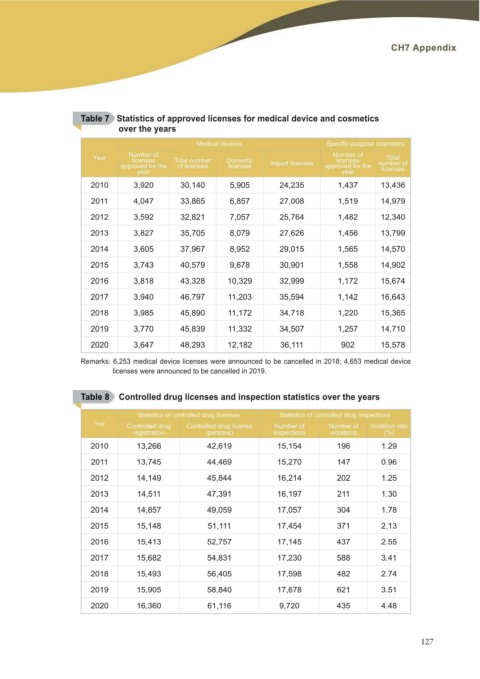
Table 7 Statistics of approved licenses for medical device and cosmetics
over the years
Medical devices 6SHFL¿F SXUSRVH FRVPHWLFV
Year Number of Total number Domestic Number of Total
licenses licenses licenses number of
approved for the of licenses Import licenses licenses
approved for the
year year
2010 3,920 30,140 5,905 24,235 1,437 13,436
2011 4,047 33,865 6,857 27,008 1,519 14,979
2012 3,592 32,821 7,057 25,764 1,482 12,340
2013 3,827 35,705 8,079 27,626 1,456 13,799
2014 3,605 37,967 8,952 29,015 1,565 14,570
2015 3,743 40,579 9,678 30,901 1,558 14,902
2016 3,818 43,328 10,329 32,999 1,172 15,674
2017 3,940 46,797 11,203 35,594 1,142 16,643
2018 3,985 45,890 11,172 34,718 1,220 15,365
2019 3,770 45,839 11,332 34,507 1,257 14,710
2020 3,647 48,293 12,182 36,111 902 15,578
Remarks: 6,253 medical device licenses were announced to be cancelled in 2018; 4,653 medical device
licenses were announced to be cancelled in 2019.
Table 8 Controlled drug licenses and inspection statistics over the years
Statistics of controlled drug licenses Statistics of controlled drug inspections
Year Controlled drug Controlled drug license Number of Number of Violation rate
registration (persons) inspections
2010 violations (%)
2011
2012 13,266 42,619 15,154 196 1.29
2013
2014 13,745 44,469 15,270 147 0.96
2015
2016 14,149 45,844 16,214 202 1.25
2017
2018 14,511 47,391 16,197 211 1.30
2019
2020 14,857 49,059 17,057 304 1.78
15,148 51,111 17,454 371 2.13
15,413 52,757 17,145 437 2.55
15,682 54,831 17,230 588 3.41
15,493 56,405 17,598 482 2.74
15,905 58,840 17,678 621 3.51
16,360 61,116 9,720 435 4.48
127