Page 119 - 2021 Taiwan Food and Drug Administration Annual Report
P. 119
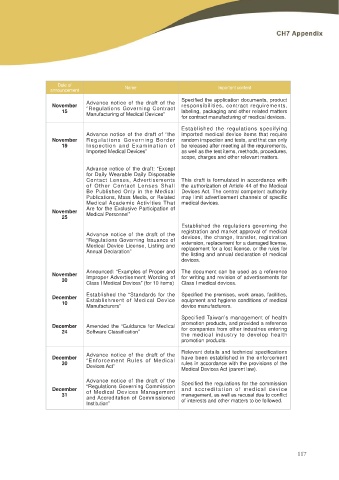
Date of Name Important content
announcement
Advance notice of the draft of the 6SHFL¿HG WKH DSSOLFDWLRQ GRFXPHQWV SURGXFW
November “Regulations Governing Contract responsibilities, contract requirements,
15 Manufacturing of Medical Devices” labeling, packaging and other related matters
for contract manufacturing of medical devices.
November Advance notice of the draft of “the Established the regulations specifying
19 Regulations Governing Border imported medical device items that require
Inspection and Examination of random inspection and tests, and that can only
Imported Medical Devices” be released after meeting all the requirements,
as well as the test items, methods, procedures,
scope, charges and other relevant matters.
Advance notice of the draft: “Except This draft is formulated in accordance with
for Daily Wearable Daily Disposable the authorization of Article 44 of the Medical
Contact Lenses, Advertisements Devices Act. The central competent authority
of Other Contact Lenses Shall may limit advertisement channels of specific
Be Published Only in the Medical medical devices.
Publications, Mass Media, or Related
November Medical Academic Activities That
25 Are for the Exclusive Participation of
Medical Personnel”
Advance notice of the draft of the Established the regulations governing the
“Regulations Governing Issuance of registration and market approval of medical
Medical Device License, Listing and devices, the change, transfer, registration
Annual Declaration” extension, replacement for a damaged license,
replacement for a lost license, or the rules for
the listing and annual declaration of medical
devices.
November Announced: “Examples of Proper and The document can be used as a reference
30 Improper Advertisement Wording of for writing and revision of advertisements for
Class I Medical Devices” (for 10 items) Class I medical devices.
December Established the “Standards for the 6SHFL¿HG WKH SUHPLVHV ZRUN DUHDV IDFLOLWLHV
10
Establishment of Medical Device equipment and hygiene conditions of medical
Manufacturers” device manufacturers.
December Amended the “Guidance for Medical Specified Taiwan’s management of health
24 6RIWZDUH &ODVVL¿FDWLRQ´ promotion products, and provided a reference
for companies from other industries entering
the medical industry to develop health
promotion products.
December Advance notice of the draft of the Relevant details and technical specifications
30 “Enforcement Rules of Medical have been established in the enforcement
Devices Act” rules in accordance with the provisions of the
Medical Devices Act (parent law).
December Advance notice of the draft of the Specified the regulations for the commission
31 “Regulations Governing Commission
of Medical Devices Management and accreditation of medical device
and Accreditation of Commissioned PDQDJHPHQW DV ZHOO DV UHFXVDO GXH WR FRQÀLFW
Institution” of interests and other matters to be followed.
117