Page 114 - 2021 Taiwan Food and Drug Administration Annual Report
P. 114
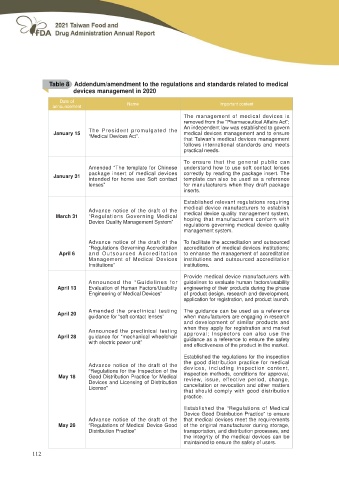
Table 8 Addendum/amendment to the regulations and standards related to medical
devices management in 2020
Date of Name Important content
announcement
January 15 The President promulgated the The management of medical devices is
“Medical Devices Act”. UHPRYHG IURP WKH ³3KDUPDFHXWLFDO $ႇDLUV $FW´
An independent law was established to govern
medical devices management and to ensure
that Taiwan’s medical devices management
follows international standards and meets
practical needs.
January 31 Amended “The template for Chinese To e n s u r e t h a t t h e g e n e r a l p u b l i c c a n
package insert of medical devices understand how to use soft contact lenses
intended for home use Soft contact correctly by reading the package insert. The
lenses” template can also be used as a reference
for manufacturers when they draft package
inserts.
March 31 Advance notice of the draft of the Established relevant regulations requiring
“Regulations Governing Medical medical device manufacturers to establish
Device Quality Management System” medical device quality management system,
hoping that manufacturers conform with
regulations governing medical device quality
management system.
April 6 Advance notice of the draft of the To facilitate the accreditation and outsourced
“Regulations Governing Accreditation accreditation of medical devices institutions;
and Outsourced Accreditation to enhance the management of accreditation
Management of Medical Devices institutions and outsourced accreditation
Institutions” institutions.
April 13 Announced the “Guidelines for Provide medical device manufacturers with
Evaluation of Human Factors/Usability guidelines to evaluate human factors/usability
Engineering of Medical Devices” engineering of their products during the phase
of product design, research and development,
application for registration, and product launch.
April 20 Amended the preclinical testing The guidance can be used as a reference
April 28 guidance for “soft contact lenses” when manufacturers are engaging in research
and development of similar products and
Announced the preclinical testing when they apply for registration and market
guidance for “mechanical wheelchair approval; Inspectors can also use the
with electric power unit” guidance as a reference to ensure the safety
DQG HႇHFWLYHQHVV RI WKH SURGXFW LQ WKH PDUNHW
May 18 Advance notice of the draft of the Established the regulations for the inspection
“Regulations for the Inspection of the the good distribution practice for medical
Good Distribution Practice for Medical devices, including inspection content,
Devices and Licensing of Distribution inspection methods, conditions for approval,
License” review, issue, effective period, change,
cancellation or revocation and other matters
that should comply with good distribution
practice.
May 26 Advance notice of the draft of the Established the “Regulations of Medical
“Regulations of Medical Device Good Device Good Distribution Practice” to ensure
Distribution Practice” that medical devices meet the requirements
of the original manufacturer during storage,
transportation, and distribution processes, and
the integrity of the medical devices can be
maintained to ensure the safety of users.
112