Page 116 - 2021 Taiwan Food and Drug Administration Annual Report
P. 116
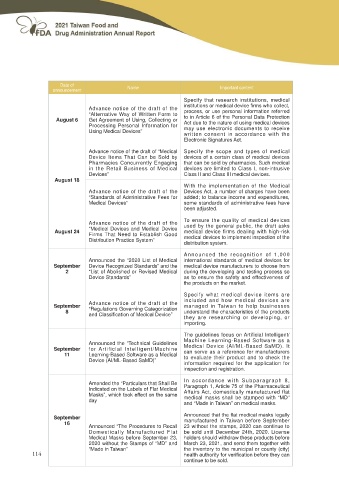
Date of Name Important content
announcement
Advance notice of the draft of the Specify that research institutions, medical
August 6 “Alternative Way of Written Form to LQVWLWXWLRQV RU PHGLFDO GHYLFH ¿UPV ZKR FROOHFW
Get Agreement of Using, Collecting or process, or use personal information referred
Processing Personal Information for to in Article 6 of the Personal Data Protection
Using Medical Devices” Act due to the nature of using medical devices
may use electronic documents to receive
written consent in accordance with the
Electronic Signatures Act.
Advance notice of the draft of “Medical Specify the scope and types of medical
Device Items That Can be Sold by devices of a certain class of medical devices
Pharmacies Concurrently Engaging that can be sold by pharmacies. Such medical
in the Retail Business of Medical devices are limited to Class I, non-intrusive
Devices” Class II and Class III medical devices.
August 18
Advance notice of the draft of the With the implementation of the Medical
“Standards of Administrative Fees for Devices Act, a number of charges have been
Medical Devices” added; to balance income and expenditures,
some standards of administrative fees have
been adjusted.
August 24 Advance notice of the draft of the To ensure the quality of medical devices
“Medical Devices and Medical Device used by the general public, the draft asks
Firms That Need to Establish Good medical device firms dealing with high-risk
Distribution Practice System” medical devices to implement inspection of the
distribution system.
September Announced the “2020 List of Medical Announced the recognition of 1,000
2 Device Recognized Standards” and the international standards of medical devices for
“List of Abolished or Revised Medical medical device manufacturers to choose from
Device Standards” during the developing and testing process so
as to ensure the safety and effectiveness of
the products on the market.
September Advance notice of the draft of the Specify what medical device items are
8 “Regulations Governing Categorization included and how medical devices are
DQG &ODVVL¿FDWLRQ RI 0HGLFDO 'HYLFH´ managed in Taiwan to help businesses
understand the characteristics of the products
they are researching or developing, or
importing.
September Announced the “Technical Guidelines The guidelines focus on Artificial Intelligent/
11 for Artificial Intelligent/Machine Machine Learning-Based Software as a
Learning-Based Software as a Medical Medical Device (AI/ML-Based SaMD). It
Device (AI/ML-Based SaMD)” can serve as a reference for manufacturers
to evaluate their product and to check the
information required for the application for
inspection and registration.
Amended the “Particulars that Shall Be In accordance with Subparagraph 8,
Paragraph 1, Article 75 of the Pharmaceutical
Indicated on the Labels of Flat Medical Affairs Act, domestically manufactured flat
0DVNV´ ZKLFK WRRN HႇHFW RQ WKH VDPH medical masks shall be stamped with “MD”
day and “Made in Taiwan” on medical masks.
September $QQRXQFHG WKDW WKH ÀDW PHGLFDO PDVNV OHJDOO\
16 manufactured in Taiwan before September
Announced “The Procedures to Recall 23 without the stamps, 2020 can continue to
Domestically Manufactured Flat be sold until December 24th, 2020. License
Medical Masks before September 23, holders should withdraw these products before
2020 without the Stamps of “MD” and March 23, 2021, and send them together with
“Made in Taiwan” the inventory to the municipal or county (city)
KHDOWK DXWKRULW\ IRU YHUL¿FDWLRQ EHIRUH WKH\ FDQ
114
continue to be sold.