Page 120 - 2021 Taiwan Food and Drug Administration Annual Report
P. 120
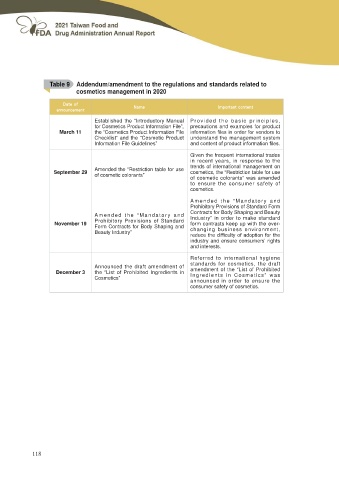
Table 9 Addendum/amendment to the regulations and standards related to
cosmetics management in 2020
March 11 Established the “Introductory Manual Provided the basic principles,
for Cosmetics Product Information File”,
the “Cosmetics Product Information File precautions and examples for product
Checklist” and the “Cosmetic Product LQIRUPDWLRQ ¿OHV LQ RUGHU IRU YHQGRUV WR
Information File Guidelines” understand the management system
DQG FRQWHQW RI SURGXFW LQIRUPDWLRQ ¿OHV
September 29 Amended the “Restriction table for use Given the frequent international trades
of cosmetic colorants” in recent years, in response to the
trends of international management on
cosmetics, the “Restriction table for use
of cosmetic colorants” was amended
to ensure the consumer safety of
cosmetics.
November 19 Amended the “Mandatory and Amended the “Mandatory and
Prohibitory Provisions of Standard Prohibitory Provisions of Standard Form
Form Contracts for Body Shaping and Contracts for Body Shaping and Beauty
Beauty Industry” Industry” in order to make standard
form contracts keep up with the ever-
changing business environment,
UHGXFH WKH GLႈFXOW\ RI DGRSWLRQ IRU WKH
industry and ensure consumers' rights
and interests.
December 3 Announced the draft amendment of Referred to international hygiene
the “List of Prohibited Ingredients in standards for cosmetics, the draft
Cosmetics” amendment of the “List of Prohibited
Ingredients in Cosmetics” was
announced in order to ensure the
consumer safety of cosmetics.
118