Page 112 - 2021 Taiwan Food and Drug Administration Annual Report
P. 112
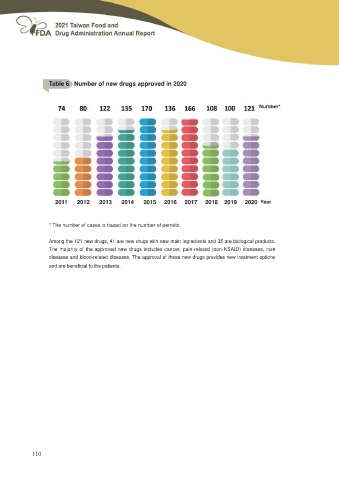
Table 6 Number of new drugs approved in 2020
Number*
2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Year
* The number of cases is based on the number of permits.
Among the 121 new drugs, 41 are new drugs with new main ingredients and 35 are biological products.
The majority of the approved new drugs includes cancer, pain-related (non-NSAID) diseases, rare
diseases and blood-related diseases. The approval of these new drugs provides new treatment options
DQG DUH EHQH¿FLDO WR WKH SDWLHQWV
110