Page 109 - 2021 Taiwan Food and Drug Administration Annual Report
P. 109
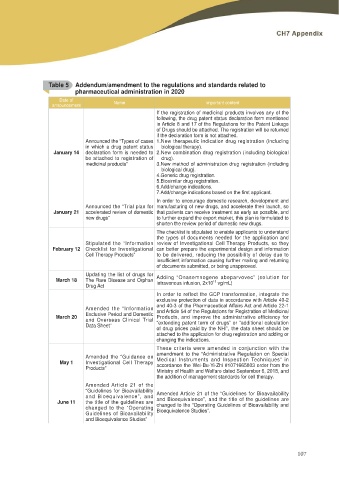
Table 5 Addendum/amendment to the regulations and standards related to
pharmaceutical administration in 2020
Date of Name Important content
announcement
If the registration of medicinal products involves any of the
following, the drug patent status declaration form mentioned
in Article 8 and 17 of the Regulations for the Patent Linkage
of Drugs should be attached. The registration will be returned
if the declaration form is not attached.
Announced the “Types of cases 1.New therapeutic indication drug registration (including
in which a drug patent status biological therapy).
January 14 declaration form is needed to 2.New combination drug registration (including biological
be attached to registration of drug).
medicinal products” 3.New method of administration drug registration (including
biological drug).
4.Generic drug registration.
5.Biosimilar drug registration.
6.Add/change indications.
$GG FKDQJH LQGLFDWLRQV EDVHG RQ WKH ¿UVW DSSOLFDQW
In order to encourage domestic research, development and
Announced the “Trial plan for manufacturing of new drugs, and accelerate their launch, so
January 21 accelerated review of domestic that patients can receive treatment as early as possible, and
new drugs” to further expand the export market, this plan is formulated to
shorten the review period of domestic new drugs.
The checklist is stipulated to enable applicants to understand
the types of documents needed for the application and
Stipulated the “Information review of Investigational Cell Therapy Products, so they
February 12 Checklist for Investigational can better prepare the experimental design and information
Cell Therapy Products” to be delivered, reducing the possibility of delay due to
LQVXႈFLHQW LQIRUPDWLRQ FDXVLQJ IXUWKHU PDLOLQJ DQG UHWXUQLQJ
of documents submitted, or being unapproved.
March 18 Updating the list of drugs for Adding “Onasemnogene abeparvovec” (solution for
The Rare Disease and Orphan intravenous infusion, 2x1013 vg/mL)
Drug Act
In order to reflect the GCP transformation, integrate the
exclusive protection of data in accordance with Article 40-2
March 20 Amended the “Information DQG RI WKH 3KDUPDFHXWLFDO $ႇDLUV $FW DQG $UWLFOH
Exclusive Period and Domestic and Article 54 of the Regulations for Registration of Medicinal
and Overseas Clinical Trial Products, and improve the administrative efficiency for
Data Sheet” “extending patent term of drugs” or “additional calculation
of drug prices paid by the NHI”, the data sheet should be
attached to the application for drug registration and adding or
changing the indications.
These criteria were amended in conjunction with the
May 1 Amended the “Guidance on amendment to the “Administrative Regulation on Special
Investigational Cell Therapy Medical Instruments and Inspection Techniques” in
Products” accordance the Wei-Bu-Yi-Zhi #1071665803 order from the
Ministry of Health and Welfare dated September 6, 2018, and
the addition of management standards for cell therapy.
Amended Article 21 of the
June 11 “Guidelines for Bioavailability Amended Article 21 of the “Guidelines for Bioavailability
and Bioequivalence”, and and Bioequivalence”, and the title of the guidelines are
the title of the guidelines are changed to the “Operating Guidelines of Bioavailability and
changed to the “Operating Bioequivalence Studies”.
Guidelines of Bioavailability
and Bioequivalence Studies”
107