Page 115 - 2018食藥署年報(英文版)
P. 115
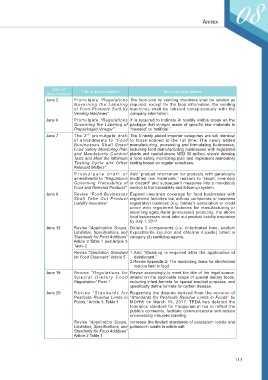
Date of
announcement Title of announcement Key point descriptions
June 2 Promulgate “Regulations The food sold by vending machines shall be labeled as
Governing the Labeling required; except for the food information, the vending
of Food Products Sold by machines shall be labeled conspicuously with the
Vending Machines” company information.
June 6 Promulgate “Regulations It is required to indicate in readily visible areas on the
Governing the Labeling of package that vinegar made of specific raw materials is
Prepackaged Vinegar” “blended” or “artificial.”
nd
June 7 The 2 promulgate draft The 5 newly added importer categories are still identical
of amendments to “Food to those noticed at the 1st time. The newly added
Businesses Shall Enact manufacturing, processing and formulating businesses,
Food Safety Monitoring Plan including food manufacturing businesses with registered
and Mandatorily Conduct plants and capital above NTD 30 million, should develop
Tests and Meet the Minimum a food safety monitoring plan and implement mandatory
Testing Cycle and Other testing based on regular schedules.
Relevant Matters”
Promulgate draft of Add “product information for products with genetically
amendments to “Regulations modified raw materoals,” reasons to “recall, inventory
Governing Traceability of or discard” and subsequent measures into a mandatory
Food and Relevant Products” section in the traceability and follow-up system.
June 8 Revise “Food Businesses Expand insurance coverage for food businesses with
Shall Take Out Product registered factories but without companies or business
Liability Insurance” registration business (e.g. farmer’s association or credit
union with registered factories for manufacturing or
importing agricultural (processed) products); the above
food businesses must take out product liability insurance
by July 1, 2017.
June 12 Revise “Application Scope, Delete 3 components (i.e. chlorinated lime, sodium
Limitation, Specifications, and hypochlorite solution and chlorine dioxide) listed in
Standards for Food Additives” category (II) sanitizing agents.
Article 2 Table 1 and Article 3
Table 2
Revise “Sanitation Standard 1.Add “Washing is required after the application of
for Food Cleansers” Article 5 disinfectant.”
2.Revise Appendix 2: The monitoring items for disinfectant
residue limit in food.
June 19 Revise “Regulations for Revise accordingly to meet the title of the legal source,
Special Dietary Food amend on the applicable scope of special dietary foods,
Registration” Point 1 including infant formula for special medical purposes, and
specifically define formula for certain disease.
June 29 Revise “St andards f or Regarding the dispute derived from the revision of
Pesticide Residue Limits in “Standards for Pesticide Residue Limits in Foods” by
Foods,” Article 3, Table 1 MOHW on March 15, 2017, TFDA has deleted the
tolerance standard for Fluopyram in tea to reflect the
public’s comments, facilitate communications and reduce
unnecessary misunderstanding.
Revise “Application Scope, Increase the limited standards of potassium iodide and
Limitation, Specifications, and potassium iodate in edible salt.
Standards for Food Additives”
Article 2 Table 1
113