Page 64 - 2017食品藥物管理署年報(英文版)
P. 64
2017 Taiwan Food and Drug Administration Annual Report
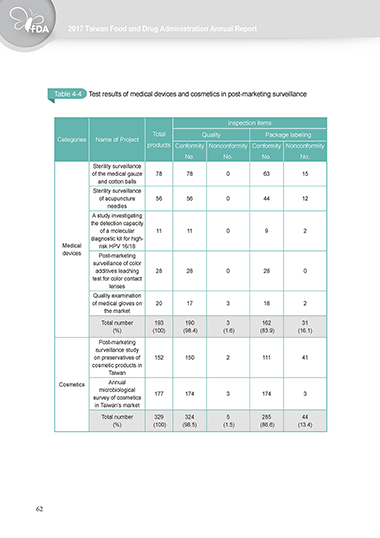
Table 4-4 Test results of medical devices and cosmetics in post-marketing surveillance
Inspection items
Total Quality Package labeling
Categories Name of Project
products Conformity Nonconformity Conformity Nonconformity
No. No. No. No.
Sterility surveillance
of the medical gauze 78 78 0 63 15
and cotton balls
Sterility surveillance
of acupuncture 56 56 0 44 12
needles
A study investigating
the detection capacity
of a molecular 11 11 0 9 2
diagnostic kit for high-
Medical risk HPV 16/18
devices Post-marketing
surveillance of color
additives leaching 28 28 0 28 0
test for color contact
lenses
Quality examination
of medical gloves on 20 17 3 18 2
the market
Total number 193 190 3 162 31
(%) (100) (98.4) (1.6) (83.9) (16.1)
Post-marketing
surveillance study
on preservatives of 152 150 2 111 41
cosmetic products in
Taiwan
Cosmetics Annual
microbiological
survey of cosmetics 177 174 3 174 3
in Taiwan’s market
Total number 329 324 5 285 44
(%) (100) (98.5) (1.5) (86.6) (13.4)
62