Page 59 - 2017食品藥物管理署年報(英文版)
P. 59
2017 Taiwan Food and Drug Administration Annual Report Chapter 4. Distribution and Post-market Management
(3) Medical devices and cosmetics
A. Medical devices and cosmetics ADR
To strengthen the practicality and convenience of reporting system, TFDA
integrates the original system and establishes “Post-market Quality Management
System for Food, Medicinal Products and Cosmetics”. TFDA received 427
medical devices adverse reactions and 3,429 defective medical devices in 2016,
which had been evaluated subsequently. A total of 58 cosmetics adverse events
were revealed in 2016, of which 15 cases of adverse reactions and 43 cases of
defective products, all have been informed to local health bureaus for related
matters or companies to submit investigation reports. TFDA also continues
follow-ups.
B. Online cosmetics safety surveillance
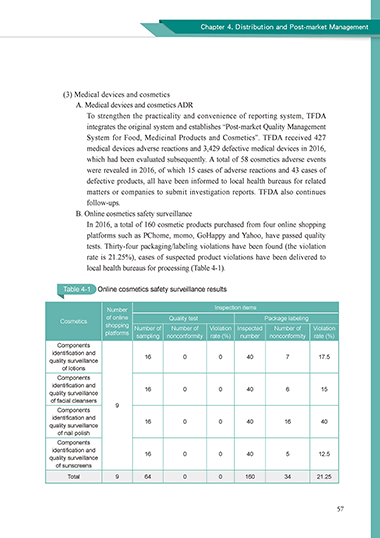
In 2016, a total of 160 cosmetic products purchased from four online shopping
platforms such as PChome, momo, GoHappy and Yahoo, have passed quality
tests. Thirty-four packaging/labeling violations have been found (the violation
rate is 21.25%), cases of suspected product violations have been delivered to
local health bureaus for processing (Table 4-1).
Table 4-1 Online cosmetics safety surveillance results
Number Inspection items
of online Quality test Package labeling
Cosmetics
shopping Number of Number of Violation Inspected Number of Violation
platforms
sampling nonconformity rate (%) number nonconformity rate (%)
Components
identification and
quality surveillance 16 0 0 40 7 17.5
of lotions
Components
identification and
quality surveillance 16 0 0 40 6 15
of facial cleansers
9
Components
identification and
quality surveillance 16 0 0 40 16 40
of nail polish
Components
identification and
quality surveillance 16 0 0 40 5 12.5
of sunscreens
Total 9 64 0 0 160 34 21.25
57