Page 60 - 2017食品藥物管理署年報(英文版)
P. 60
2017 Taiwan Food and Drug Administration Annual Report
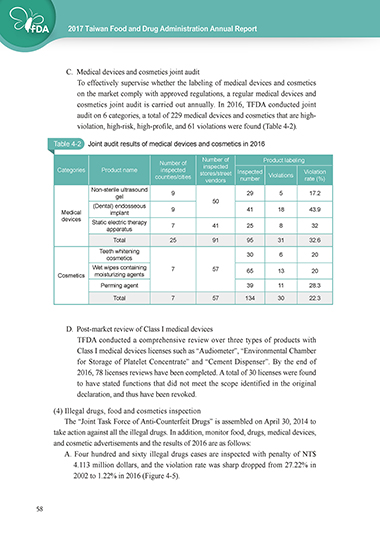
C. Medical devices and cosmetics joint audit
To effectively supervise whether the labeling of medical devices and cosmetics
on the market comply with approved regulations, a regular medical devices and
cosmetics joint audit is carried out annually. In 2016, TFDA conducted joint
audit on 6 categories, a total of 229 medical devices and cosmetics that are high-
violation, high-risk, high-profile, and 61 violations were found (Table 4-2).
Table 4-2 Joint audit results of medical devices and cosmetics in 2016
Number of Product labeling
Number of
Categories Product name inspected inspected Inspected Violation
counties/cities stores/street Violations
vendors number rate (%)
Non-sterile ultrasound 9 29 5 17.2
gel 50
(Dental) endosseous
Medical implant 9 41 18 43.9
devices
Static electric therapy 7 41 25 8 32
apparatus
Total 25 91 95 31 32.6
Teeth whitening
cosmetics 30 6 20
Wet wipes containing 7 57 65 13 20
Cosmetics moisturizing agents
Perming agent 39 11 28.3
Total 7 57 134 30 22.3
D. Post-market review of Class I medical devices
TFDA conducted a comprehensive review over three types of products with
Class I medical devices licenses such as “Audiometer”, “Environmental Chamber
for Storage of Platelet Concentrate” and “Cement Dispenser”. By the end of
2016, 78 licenses reviews have been completed. A total of 30 licenses were found
to have stated functions that did not meet the scope identified in the original
declaration, and thus have been revoked.
(4) Illegal drugs, food and cosmetics inspection
The “Joint Task Force of Anti-Counterfeit Drugs” is assembled on April 30, 2014 to
take action against all the illegal drugs. In addition, monitor food, drugs, medical devices,
and cosmetic advertisements and the results of 2016 are as follows:
A. Four hundred and sixty illegal drugs cases are inspected with penalty of NT$
4.113 million dollars, and the violation rate was sharp dropped from 27.22% in
2002 to 1.22% in 2016 (Figure 4-5).
58