Page 63 - 2017食品藥物管理署年報(英文版)
P. 63
2017 Taiwan Food and Drug Administration Annual Report Chapter 4. Distribution and Post-market Management
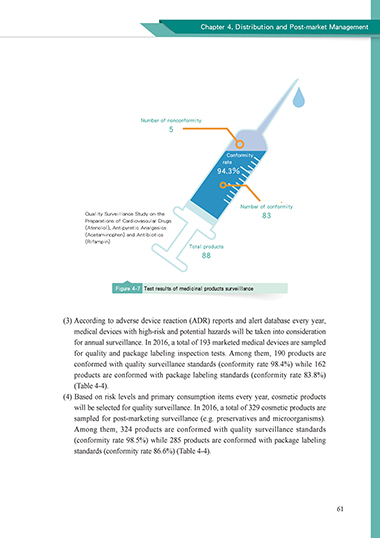
Number of nonconformity
5
Conformity
rate
94.3%
Number of conformity
Quality Surveillance Study on the 83
Preparations of Cardiovascular Drugs
(Atenolol), Antipyretic Analgesics
(Acetaminophen) and Antibiotics
(Rifampin)
Total products
88
Figure 4-7 Test results of medicinal products surveillance
(3) According to adverse device reaction (ADR) reports and alert database every year,
medical devices with high-risk and potential hazards will be taken into consideration
for annual surveillance. In 2016, a total of 193 marketed medical devices are sampled
for quality and package labeling inspection tests. Among them, 190 products are
conformed with quality surveillance standards (conformity rate 98.4%) while 162
products are conformed with package labeling standards (conformity rate 83.8%)
(Table 4-4).
(4) Based on risk levels and primary consumption items every year, cosmetic products
will be selected for quality surveillance. In 2016, a total of 329 cosmetic products are
sampled for post-marketing surveillance (e.g. preservatives and microorganisms).
Among them, 324 products are conformed with quality surveillance standards
(conformity rate 98.5%) while 285 products are conformed with package labeling
standards (conformity rate 86.6%) (Table 4-4).
61