Page 15 - 2017食品藥物管理署年報(英文版)
P. 15
2017 Taiwan Food and Drug Administration Annual Report Chapter 1. Management Overview
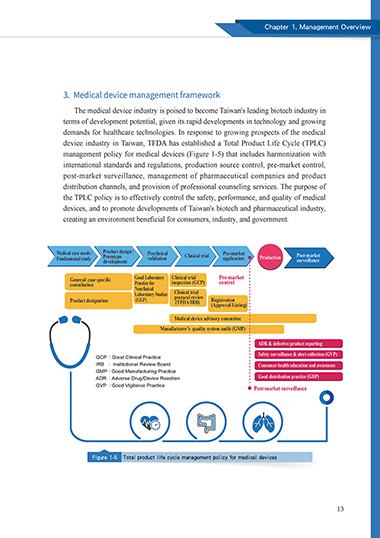
3. Medical device management framework
The medical device industry is poised to become Taiwan's leading biotech industry in
terms of development potential, given its rapid developments in technology and growing
demands for healthcare technologies. In response to growing prospects of the medical
device industry in Taiwan, TFDA has established a Total Product Life Cycle (TPLC)
management policy for medical devices (Figure 1-5) that includes harmonization with
international standards and regulations, production source control, pre-market control,
post-market surveillance, management of pharmaceutical companies and product
distribution channels, and provision of professional counseling services. The purpose of
the TPLC policy is to effectively control the safety, performance, and quality of medical
devices, and to promote developments of Taiwan's biotech and pharmaceutical industry,
creating an environment beneficial for consumers, industry, and government.
Medical care needs / Product design / Preclinical Clinical trial Pre-market Post-market
Fundamental study Prototype validation application Production
development surveillance
General/ case specific Good Laboratory Clinical trial Pre-market
consultation Practice for inspection (GCP) control
Nonclinical
Laboratory Studies Clinical trial
Product designation (GLP) protocol review Registration
(TFDA/IRB)
(Approval/Listing)
Medical device advisory committee
Manufacturer’s quality system audit (GMP)
ADR & defective product reporting
Safety surveillance & alert collection (GVP)
GCP :Good Clinical Practice
IRB : Institutional Review Board Consumer health education and awareness
GMP:Good Manufacturing Practice
ADR :Adverse Drug/Device Reaction Good distribution practice (GDP)
GVP :Good Vigilance Practice
Post-market surveillance
Figure 1-5 Total product life cycle management policy for medical devices
13