Page 124 - 2017食品藥物管理署年報(英文版)
P. 124
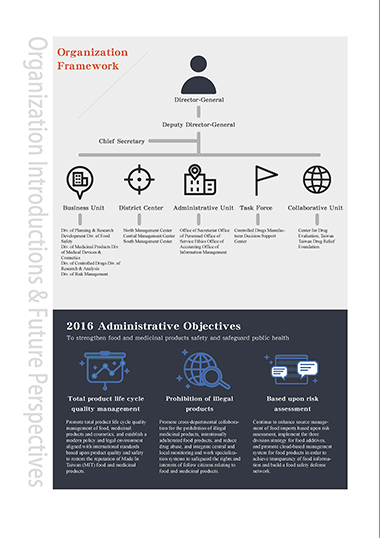
Organization
Framework
Director-General
Deputy Director-General
Chief Secretary
Business Unit District Center Administrative Unit Task Force Collaborative Unit
Div. of Planning & Research North Management Center Office of Secretariat Office Controlled Drugs Manufac- Center for Drug
Development Div. of Food Central Management Center of Personnel Office of turer Decision Support Evaluation, Taiwan
Safety South Management Center Service Ethics Office of Center Taiwan Drug Relief
Div. of Medicinal Products Div. Accounting Office of Foundation
of Medical Devices & Information Management
Cosmetics
Div. of Controlled Drugs Div. of
Research & Analysis
Div. of Risk Management
2016 Administrative Objectives
To strengthen food and medicinal products safety and safeguard public health
Total product life cycle Prohibition of illegal Based upon risk
quality management products assessment
Promote total product life cycle quality Promote cross-departmental collabora- Continue to enhance source manage-
management of food, medicinal tion for the prohibition of illegal ment of food imports based upon risk
products and cosmetics, and establish a medicinal products, intentionally assessment, implement the three
modern policy and legal environment adulterated food products, and reduce division strategy for food additives,
aligned with international standards drug abuse, and integrate central and and promote cloud-based management
based upon product quality and safety local monitoring and work specializa- system for food products in order to
to restore the reputation of Made In tion systems to safeguard the rights and achieve transparency of food informa-
Taiwan (MIT) food and medicinal interests of fellow citizens relating to tion and build a food safety defense
products. food and medicinal products. network.
Organization Introductions & Future Perspectives