Page 53 - Taiwan Food and Drug Administration 2016 Annual Report
P. 53
2016 ANNUAL
REPORT
(days)
(3) Establishing a Quality Environment for 54
55
Medical Device Clinical Trial Regulations
50
a. In 2015, TFDA promulgated the Good Clinical
45 42
Practice for Medical Devices (GCP) that 38
40
was harmonized with ISO 14155:2011 and 34
revised the Application Notes for Medical 35
Device Clinical Trial Protocols, providing a 30
reference for manufacturers and hospitals 25
intending to conduct clinical trials of medical 20 Part II - Key Administrative Results Medical Devices Management
devices. 15
10
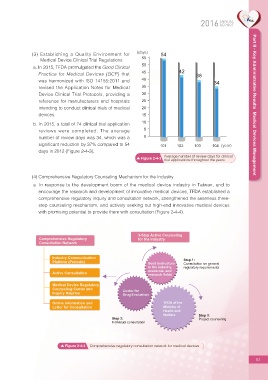
b. In 2015, a total of 74 clinical trial application
reviews were completed. The average 5
number of review days was 34, which was a 0
signi?cant reduction by 37% compared to 54 101 102 103 104 (year)
days in 2012 (Figure 2-4-3).
Average number of review days for clinical
Figure 2-4-3
trial applications throughout the years
(4) Comprehensive Regulatory Counseling Mechanism for the Industry
a. In response to the development boom of the medical device industry in Taiwan, and to
encourage the research and development of innovative medical devices, TFDA established a
comprehensive regulatory inquiry and consultation network, strengthened the seamless three-
step counseling mechanism, and actively seeking out high-end innovative medical devices
with promising potential to provide them with consultation (Figure 2-4-4).
3-Step Active Counseling
Comprehensive Regulatory for the Industry
Consultation Network
Industry Communication
Platform (Periodic) Seed instructors Step 1:
Consultation on general
in the industry, regulatory requirements
academia, and
Active Consultation research fields
Medical Device Regulatory
Counseling Center and Center for
Inquiry Helpline Drug Evaluation
Online Information and TFDA of the
Letter for Consultation Ministry of
Health and
Welfare Step 3:
Step 2: Project counseling
Individual consultation
Figure 2-4-4 Comprehensive regulatory consultation network for medical devices
51