Page 56 - Taiwan Food and Drug Administration 2016 Annual Report
P. 56
Taiwan Food and Drug Adminstration
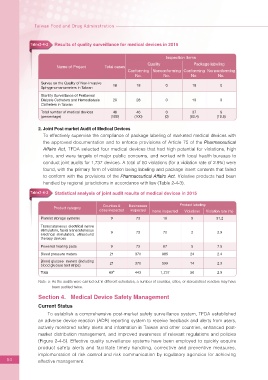
Table2-4-2 Results of quality surveillance for medical devices in 2015
Inspection Items
Quality Package labeling
Name of Project Total cases
Conforming Nonconforming Conforming Nonconforming
No. No. No. No.
Survey on the Quality of Non-Invasive 18 18 0 18 0
Sphygmomanometers in Taiwan
Sterility Surveillance of Peritoneal
Dialysis Catheters and Hemodialysis 28 28 0 19 9
Catheters in Taiwan
Total number of medical devices 46 46 0 37 9
(percentage) (100) (100) (0) (80.4) (19.6)
2. Joint Post-market Audit of Medical Devices
To effectively supervise the compliance of package labeling of marketed medical devices with
the approved documentation and to enforce provisions of Article 75 of the Pharmaceutical
Affairs Act, TFDA selected four medical devices that had high potential for violations, high
risks, and were targets of major public concerns, and worked with local health bureaus to
conduct joint audits for 1,737 devices. A total of 50 violations (for a violation rate of 2.9%) were
found, with the primary form of violation being labeling and package insert contents that failed
to conform with the provisions of the Pharmaceutical Affairs Act. Violative products had been
handled by regional jurisdictions in accordance with law (Table 2-4-3).
Table2-4-3 Statistical analysis of joint audit results of medical devices in 2015
Counties & Businesses Product labeling
Product category
cities inspected inspected Items inspected Violations Violation rate (%)
Platelet storage systems 9 73 16 5 31.2
Transcutaneous electrical nerve
stimulators, facial transcutaneous 9 73 70 2 2.9
electrical stimulators, ultrasound
therapy devices
Powered heating pads 9 73 67 5 7.5
Blood pressure meters 21 370 985 24 2.4
Blood glucose meters (including
blood glucose test strips) 21 370 599 14 2.3
a
Total 69 443 1,737 50 2.9
Note: a: As the audits were carried out in different schedules, a number of counties, cities, or stores/street vendors may have
been audited twice.
Section 4. Medical Device Safety Management
Current Status
To establish a comprehensive post-market safety surveillance system, TFDA established
an adverse device reaction (ADR) reporting system to receive feedback and alerts from users,
actively monitored safety alerts and information in Taiwan and other countries, enhanced post-
market distribution management, and improved awareness of relevant regulations and policies
(Figure 2-4-5). Effective quality surveillance systems have been employed to quickly acquire
product safety alerts and facilitate timely handling, corrective and preventive measures,
implementation of risk control and risk communication by regulatory agencies for achieving
54 effective management.