Page 118 - Taiwan Food and Drug Administration 2016 Annual Report
P. 118
Taiwan Food and Drug Adminstration
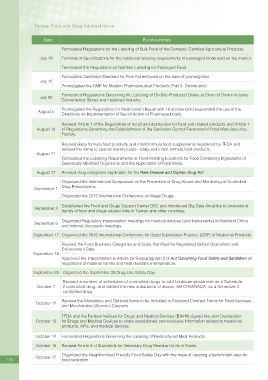
Date Event summary
Formulated Regulations for the Labeling of Bulk Food of the Domestic Certi?ed Agricultural Products.
July 10 Terminated Speci?cations for the nutritional labeling requirements of packaged foods sold on the market.
Terminated the Regulations on Nutrition Labeling for Packaged Food.
Formulated Sanitation Standard for Pork Fat enforced on the date of promulgation.
July 16
Promulgated the GMP for Modern Pharmaceutical Products (Part 3: Distribution).
Formulated Regulations Governing the Labeling of On-Site-Produced Drinks at Chain of Drinks Industry
July 20
Convenience Stores and Fast-food Industry.
Promulgated the Regulations for Medicament Recall with 19 articles (and suspended the use of the
August 5
Directions on Implementation of Recall Action of Pharmaceuticals).
Revised Article 1 of the Regulations of recall and destruction for food and related products and Article 1
August 10 of Regulations Governing the Establishment of the Sanitation Control Personnel of Food Manufacturing
Factory.
Revised Baby formula food products and infant formula food supplements registered by TFDA and
revised the name to special dietary foods - baby and infant formula food products.
August 11
Formulated the Labelling Requirements at Food Vending Locations for Food Containing Ingredients of
Genetically Modi?ed Organisms and the Application of Food Items.
August 17 Revised drug categories applicable for the Rare Disease and Orphan Drug Act.
Organized the International Symposium on the Prevention of Drug Abuse and Monitoring of Controlled
Drug Prescriptions.
September 1
Organized the 2015 International Conference on Illegal Drugs.
Established the Food and Drugs Support Center DSC and introduced Big Data Analytics to understand
September 2
trends of food and drugs related risks in Taiwan and other countries.
Organized Regulatory interpretation meetings for medical devices (and instruments) in Mainland China
September 8
and internal discussion meetings.
September 17 Organized the 2015 International Conference for Good Submission Practice (GSP) of Medicinal Products
Revised the Food Business Categories and Scale that Must be Registered Before Operations and
Enforcement Date.
September 18
Approved the interpretation to Article 26 Subparagraph 2 of Act Governing Food Safety and Sanitation on
regulations of material names and heat resistance temperature.
September 25 Organized the September 25 Drug Use Safety Day.
Revised a number of schedules of controlled drugs to add Lisdexamphetamine as a Schedule
October 7 2 controlled drug, and added the new substance of abuse, AB-CHMINACA, as a Schedule 3
controlled drug.
Revised the Mandatory and Optional Items to be Included in Standard Contract Terms for Food Services
October 12
and Merchandise (Service) Coupons.
TFDA and the Federal Institute for Drugs and Medical Devices (BfArM) signed the Joint Declaration
October 13 for Drugs and Medical Devices to share unpublished and exclusive information related to medicinal
products, APIs, and medical devices.
October 14 Formulated Regulations Governing the Labeling of Restructured Meat Products.
October 16 Revised Article 3 of Standards for Veterinary Drug Residue Limits in Foods.
Organized the Neighborhood Friendly Food Safety Day with the hope of creating a benchmark area for
October 17
116 food sanitation.