Page 135 - 2020Taiwan Food and Drug Administration Annual Report
P. 135
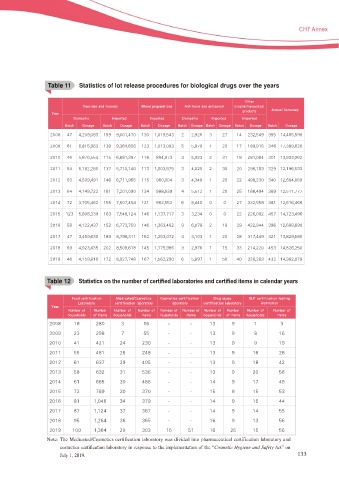
T able 1 1
Table 11 cStatistics of lot release procedures for biological drugs over the years
0UIFS
7BDDJOFT BOE UPYPJET #MPPE QSFQBSBUJPOT "OUJUPYJO BOE BOUJTFSVN CJPQIBSNBDFVUJDBM
QSPEVDUT "OOVBM 4VNNBSZ
:FBS
%PNFTUJD *NQPSUFE *NQPSUFE %PNFTUJD *NQPSUFE *NQPSUFE
#BUDI %PTBHF #BUDI %PTBHF #BUDI %PTBHF #BUDI %PTBHF #BUDI %PTBHF #BUDI %PTBHF #BUDI %PTBHF
Table 12 c6WDWLVWLFV RQ WKH QXPEHU RI FHUWL¿HG ODERUDWRULHV DQG FHUWL¿HG LWHPV LQ FDOHQGDU \HDUV
T able 12
'PPE DFSUJGJDBUJPO .FEJDBUFE $PTNFUJDT $PTNFUJDT DFSUJGJDBUJPO %SVH BCVTF (-1 DFSUJGJDBUJPO UFTUJOH
-BCPSBUPSZ DFSUJGJDBUJPO MBCPSBUPSZ MBCPSBUPSZ DFSUJGJDBUJPO MBCPSBUPSZ JOTUJUVUJPO
:FBS
/VNCFS PG /VNCFS /VNCFS PG /VNCFS PG /VNCFS PG /VNCFS PG /VNCFS PG /VNCFS /VNCFS PG /VNCFS PG
IPVTFIPMET PG JUFNT IPVTFIPMET JUFNT IPVTFIPMET JUFNT IPVTFIPMET PG JUFNT IPVTFIPMET JUFNT
Note: The Medicated/Cosmetics certification laboratory was divided into pharmaceutical certification laboratory and
FRVPHWLFV FHUWL¿FDWLRQ ODERUDWRU\ LQ UHVSRQVH WR WKH LPSOHPHQWDWLRQ RI WKH ³Cosmetic Hygiene and Safety Act” on
July 1, 2019. 133