Page 134 - 2020Taiwan Food and Drug Administration Annual Report
P. 134
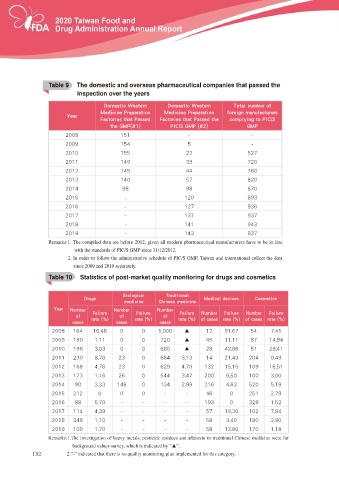
Table 9c The domestic and overseas pharmaceutical companies that passed the
T able 9
inspection over the years
%PNFTUJD 8FTUFSO %PNFTUJD 8FTUFSO 5PUBM OVNCFS PG
.FEJDJOF 1SFQBSBUJPO .FEJDJOF 1SFQBSBUJPO GPSFJHO NBOVGBDUVSFST
:FBS
'BDUPSJFT UIBU 1BTTFE 'BDUPSJFT UIBU 1BTTFE UIF DPNQMZJOH UP 1*$ 4
UIF (.1 1*$ 4 (.1 (.1
Remarks:1. The compiled data are before 2012, given all modern pharmaceutical manufacturers have to be in line
with the standards of PIC/S GMP since 31/12/2012.
2. In order to follow the administrative schedule of PIC/S GMP, Taiwan and international collect the data
since 2009 and 2010 separately.
Table 10 cStatistics of post-market quality monitoring for drugs and cosmetics
T able 10
#JPMPHJDBM 5SBEJUJPOBM
%SVHT .FEJDBM EFWJDFT $PTNFUJDT
NFEJDJOF $IJOFTF NFEJDJOF
:FBS /VNCFS /VNCFS /VNCFS
'BJMVSF 'BJMVSF 'BJMVSF /VNCFS 'BJMVSF /VNCFS 'BJMVSF
PG PG PG
SBUF SBUF SBUF PG DBTFT SBUF PG DBTFT SBUF
DBTFT DBTFT DBTFT
·
·
·
5HPDUNV 7KH LQYHVWLJDWLRQ RI KHDY\ PHWDOV SHVWLFLGH UHVLGXHV DQG DÀDWR[LQ LQ WUDGLWLRQDO &KLQHVH PHGLFLQH ZHUH IRU
background values survey, which is indicated by “Ÿ´
132 2.“-” indicated that there is no quality monitoring plan implemented for this category.