Page 126 - 2020Taiwan Food and Drug Administration Annual Report
P. 126
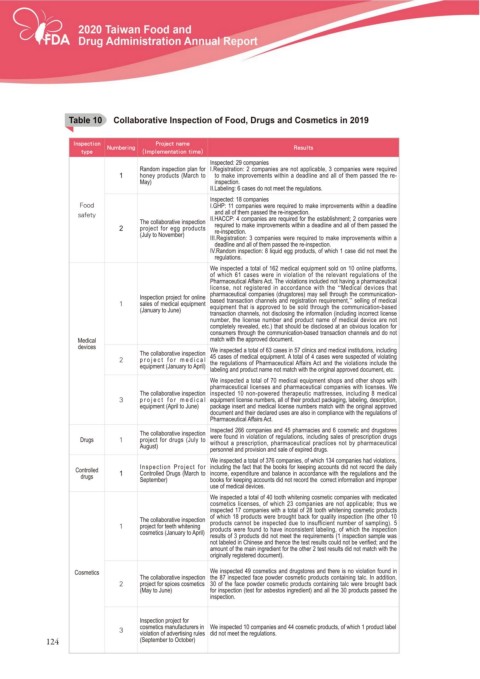
Table 10c Collaborative Inspection of Food, Drugs and Cosmetics in 2019
T able 10
*OTQFDUJPO /VNCFSJOH 1SPKFDU OBNF 3FTVMUT
UZQF *NQMFNFOUBUJPO UJNF
Inspected: 29 companies
Random inspection plan for I.Registration: 2 companies are not applicable, 3 companies were required
1 honey products (March to to make improvements within a deadline and all of them passed the re-
May) inspection.
II.Labeling: 6 cases do not meet the regulations.
Inspected: 18 companies
'PPE I.GHP: 11 companies were required to make improvements within a deadline
and all of them passed the re-inspection.
TBGFUZ II.HACCP: 4 companies are required for the establishment; 2 companies were
The collaborative inspection
2 project for egg products required to make improvements within a deadline and all of them passed the
re-inspection.
(July to November) III.Registration: 3 companies were required to make improvements within a
deadline and all of them passed the re-inspection.
IV.Random inspection: 8 liquid egg products, of which 1 case did not meet the
regulations.
We inspected a total of 162 medical equipment sold on 10 online platforms,
of which 61 cases were in violation of the relevant regulations of the
Pharmaceutical Affairs Act. The violations included not having a pharmaceutical
license, not registered in accordance with the “Medical devices that
pharmaceutical companies (drugstores) may sell through the communication-
Inspection project for online based transaction channels and registration requirement,” selling of medical
sales of medical equipment equipment that is approved to be sold through the communication-based
(January to June)
transaction channels, not disclosing the information (including incorrect license
number, the license number and product name of medical device are not
completely revealed, etc.) that should be disclosed at an obvious location for
consumers through the communication-based transaction channels and do not
Medical match with the approved document.
devices We inspected a total of 63 cases in 57 clinics and medical institutions, including
The collaborative inspection 45 cases of medical equipment. A total of 4 cases were suspected of violating
project for medical the regulations of Pharmaceutical Affairs Act and the violations include the
equipment (January to April) labeling and product name not match with the original approved document, etc.
We inspected a total of 70 medical equipment shops and other shops with
pharmaceutical licenses and pharmaceutical companies with licenses. We
The collaborative inspection inspected 10 non-powered therapeutic mattresses, including 8 medical
project for medical equipment license numbers, all of their product packaging, labeling, description,
equipment (April to June) package insert and medical license numbers match with the original approved
document and their declared uses are also in compliance with the regulations of
Pharmaceutical Affairs Act.
Inspected 266 companies and 45 pharmacies and 6 cosmetic and drugstores
The collaborative inspection
Drugs project for drugs (July to were found in violation of regulations, including sales of prescription drugs
without a prescription, pharmaceutical practices not by pharmaceutical
August)
personnel and provision and sale of expired drugs.
We inspected a total of 376 companies, of which 134 companies had violations,
Controlled 1 Inspection Project for including the fact that the books for keeping accounts did not record the daily
Controlled Drugs (March to income, expenditure and balance in accordance with the regulations and the
drugs September) books for keeping accounts did not record the correct information and improper
use of medical devices.
We inspected a total of 40 tooth whitening cosmetic companies with medicated
cosmetics licenses, of which 23 companies are not applicable; thus we
inspected 17 companies with a total of 28 tooth whitening cosmetic products
of which 18 products were brought back for quality inspection (the other 10
The collaborative inspection products cannot be inspected due to insufficient number of sampling). 5
project for teeth whitening products were found to have inconsistent labeling, of which the inspection
cosmetics (January to April)
results of 3 products did not meet the requirements (1 inspection sample was
QRW ODEHOHG LQ &KLQHVH DQG WKHQFH WKH WHVW UHVXOWV FRXOG QRW EH YHUL¿HG DQG WKH
amount of the main ingredient for the other 2 test results did not match with the
originally registered document).
Cosmetics We inspected 49 cosmetics and drugstores and there is no violation found in
The collaborative inspection the 87 inspected face powder cosmetic products containing talc. In addition,
project for spices cosmetics 30 of the face powder cosmetic products containing talc were brought back
(May to June) for inspection (test for asbestos ingredient) and all the 30 products passed the
inspection.
Inspection project for
cosmetics manufacturers in We inspected 10 companies and 44 cosmetic products, of which 1 product label
violation of advertising rules did not meet the regulations.
124 (September to October)