Page 121 - 2020Taiwan Food and Drug Administration Annual Report
P. 121
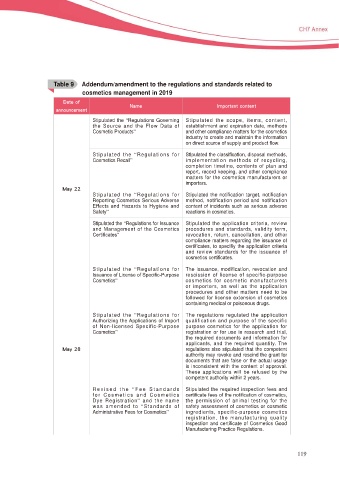
Table 9c Addendum/amendment to the regulations and standards related to
T able 9
cosmetics management in 2019
%BUF PG
/BNF *NQPSUBOU DPOUFOU
BOOPVODFNFOU
Stipulated the “Regulations Governing Stipulated the scope, items, content,
the Source and the Flow Data of HVWDEOLVKPHQW DQG H[SLUDWLRQ GDWH PHWKRGV
Cosmetic Products” and other compliance matters for the cosmetics
industry to create and maintain the information
RQ GLUHFW VRXUFH RI VXSSO\ DQG SURGXFW ÀRZ
Stipulated the “Regulations for 6WLSXODWHG WKH FODVVL¿FDWLRQ GLVSRVDO PHWKRGV
Cosmetics Recall” implementation methods of recycling,
completion timeline, contents of plan and
report, record keeping, and other compliance
matters for the cosmetics manufacturers or
importers.
.BZ
Stipulated the “Regulations for Stipulated the notification target, notification
Reporting Cosmetics Serious Adverse method, notification period and notification
(IIHFWV DQG +D]DUGV WR +\JLHQH DQG content of incidents such as serious adverse
Safety” reactions in cosmetics.
Stipulated the “Regulations for Issuance Stipulated the application criteria, review
and Management of the Cosmetics procedures and standards, validity term,
&HUWL¿FDWHV” revocation, return, cancellation, and other
compliance matters regarding the issuance of
certificates, to specifiy the application criteria
and review standards for the issuance of
FRVPHWLFV FHUWL¿FDWHV
Stipulated the “Regulations for The issuance, modification, revocation and
,VVXDQFH RI /LFHQVH RI 6SHFL¿F 3XUSRVH rescission of license of specific-purpose
Cosmetics” cosmetics for cosmetic manufacturers
or importers, as well as the application
procedures and other matters need to be
IROORZHG IRU OLFHQVH H[WHQVLRQ RI FRVPHWLFV
containing medical or poisonous drugs.
Stipulated the “Regulations for The regulations regulated the application
$XWKRUL]LQJ WKH $SSOLFDWLRQV RI ,PSRUW TXDOLILFDWLRQ DQG SXUSRVH RI WKH VSHFLILF
of Non-licensed Specific-Purpose purpose cosmetics for the application for
Cosmetics” registration or for use in research and trial,
WKH UHTXLUHG GRFXPHQWV DQG LQIRUPDWLRQ IRU
DSSOLFDQWV DQG WKH UHTXLUHG TXDQWLW\ 7KH
.BZ regulations also stipulated that the competent
authority may revoke and rescind the grant for
documents that are false or the actual usage
is inconsistent with the content of approval.
These applications will be refused by the
competent authority within 2 years.
Revised the “ Fee Standards 6WLSXODWHG WKH UHTXLUHG LQVSHFWLRQ IHHV DQG
for Cosmetics and Cosmetics FHUWL¿FDWH IHHV RI WKH QRWL¿FDWLRQ RI FRVPHWLFV
Dye Registration” and the name the permission of animal testing for the
was amended to “Standards of safety assessment of cosmetics or cosmetic
Administrative Fees for Cosmetics” ingredients, specific-purpose cosmetics
UHJLVWUDWLRQ WKH PDQXIDFWXULQJ TXDOLW\
inspection and certificate of Cosmetics Good
Manufacturing Practice Regulations.
119