Page 75 - 2017食品藥物管理署年報(英文版)
P. 75
2017 Taiwan Food and Drug Administration Annual Report Chapter 5. Testing Technology and Capability
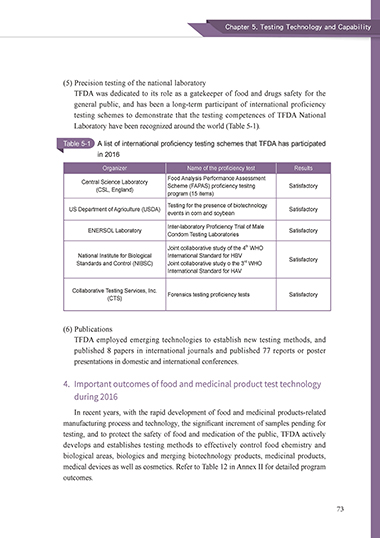
(5) Precision testing of the national laboratory
TFDA was dedicated to its role as a gatekeeper of food and drugs safety for the
general public, and has been a long-term participant of international proficiency
testing schemes to demonstrate that the testing competences of TFDA National
Laboratory have been recognized around the world (Table 5-1).
Table 5-1 A list of international proficiency testing schemes that TFDA has participated
in 2016
Organizer Name of the proficiency test Results
Food Analysis Performance Assessment
Central Science Laboratory Scheme (FAPAS) proficiency tesitng Satisfactory
(CSL, England)
program (15 items)
Testing for the presence of biotechnology
US Department of Agriculture (USDA) Satisfactory
events in corn and soybean
Inter-laboratory Proficiency Trial of Male
ENERSOL Laboratory Satisfactory
Condom Testing Laboratories
th
Joint collaborative study of the 4 WHO
National Institute for Biological International Standard for HBV
rd
Standards and Control (NIBSC) Joint collaborative study o the 3 WHO Satisfactory
International Standard for HAV
Collaborative Testing Services, Inc. Forensics testing proficiency tests Satisfactory
(CTS)
(6) Publications
TFDA employed emerging technologies to establish new testing methods, and
published 8 papers in international journals and published 77 reports or poster
presentations in domestic and international conferences.
4. Important outcomes of food and medicinal product test technology
during 2016
In recent years, with the rapid development of food and medicinal products-related
manufacturing process and technology, the significant increment of samples pending for
testing, and to protect the safety of food and medication of the public, TFDA actively
develops and establishes testing methods to effectively control food chemistry and
biological areas, biologics and merging biotechnology products, medicinal products,
medical devices as well as cosmetics. Refer to Table 12 in Annex II for detailed program
outcomes.
73