Page 80 - 2017食品藥物管理署年報(英文版)
P. 80
2017 Taiwan Food and Drug Administration Annual Report
1100
Food Medicinal products/ 1046
Cosmetics
1000
Abuse GLP GLP
900
800 789
Number of Certified Items 481
700
600 637 632 665
500 421 405 536 488
400
300 251 280 280 280 298 370 379
248
200 230
55 58
100 16 19 26 42 49 53
4 7 7 7 7 7 7 9 GLP 16 GLP GLP GLP GLP GLP GLP GLP 44
0 GLP GLP GLP GLP GLP GLP GLP GLP GLP 9 9
0 0 0 0 0 0 0 0 9 3 9 9 9 9 9 9
89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 Year
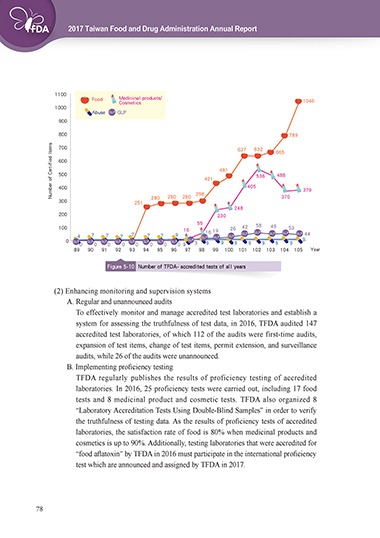
Figure 5-10 Number of TFDA- accredited tests of all years
(2) Enhancing monitoring and supervision systems
A. Regular and unannounced audits
To effectively monitor and manage accredited test laboratories and establish a
system for assessing the truthfulness of test data, in 2016, TFDA audited 147
accredited test laboratories, of which 112 of the audits were first-time audits,
expansion of test items, change of test items, permit extension, and surveillance
audits, while 26 of the audits were unannounced.
B. Implementing proficiency testing
TFDA regularly publishes the results of proficiency testing of accredited
laboratories. In 2016, 25 proficiency tests were carried out, including 17 food
tests and 8 medicinal product and cosmetic tests. TFDA also organized 8
“Laboratory Accreditation Tests Using Double-Blind Samples” in order to verify
the truthfulness of testing data. As the results of proficiency tests of accredited
laboratories, the satisfaction rate of food is 80% when medicinal products and
cosmetics is up to 90%. Additionally, testing laboratories that were accredited for
“food aflatoxin” by TFDA in 2016 must participate in the international proficiency
test which are announced and assigned by TFDA in 2017.
78