Page 79 - 2017食品藥物管理署年報(英文版)
P. 79
2017 Taiwan Food and Drug Administration Annual Report Chapter 5. Testing Technology and Capability
(1) Expanding testing capacities of laboratories
A. Accreditation programs for food laboratories as well as medicinal products and
cosmetic laboratories were initiated in 2004 and 2008 respectively. In 2010,
TFDA streamlined and expanded the laboratory accreditation action plan and
continued to promote border inspections for food and traditional Chinese
medicine, establish quantity limit standards for traditional Chinese medicine, and
provide accreditation to commissioned tests for administrative processes in order
to accelerate the expansion of testing capacities.
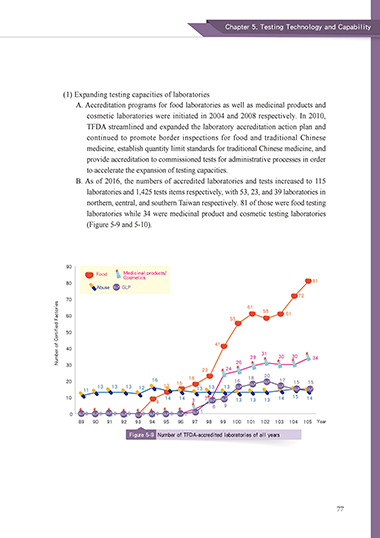
B. As of 2016, the numbers of accredited laboratories and tests increased to 115
laboratories and 1,425 tests items respectively, with 53, 23, and 39 laboratories in
northern, central, and southern Taiwan respectively. 81 of those were food testing
laboratories while 34 were medicinal product and cosmetic testing laboratories
(Figure 5-9 and 5-10).
90
Food Medicinal products/
Cosmetics
80 81
Abuse GLP GLP
72
70 61 58 61
Number of Certified Factories 50 55
60
40 41 31
30
23 24 26 29 30 30 34
20
20 16 15 18 16 18 GLP 17 15 15
GLP
13 13 13 12 13 13 13 GLP GLP
11 13 GLP GLP
10 14 14 3 7 GLP GLP 13 13 13 14 15 14
9
8 9
0 GLP 0 GLP 0 GLP 0 GLP 0 GLP 0 GLP 0 GLP 0 GLP 0 GLP 1
89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 Year
Figure 5-9 Number of TFDA-accredited laboratories of all years
77