Page 70 - 2017食品藥物管理署年報(英文版)
P. 70
2017 Taiwan Food and Drug Administration Annual Report
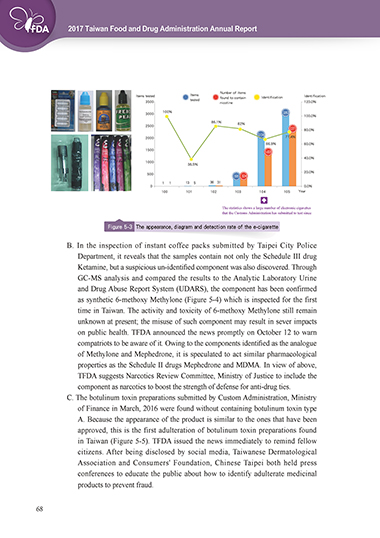
Number of items
Items tested Items found to contain Identification Identification
tested
3500 nicotine 120.0%
100% 3,062
3000
100.0%
86.1%
2500 82% 2,371
80.0%
2,134 77.4%
2000
66.9% 60.0%
1500 1,428
40.0%
1000
38.5%
500 20.0%
395 324
1 1 13 5 36 31
0 0.0%
100 101 102 103 104 105 Year
The statistics shows a large number of electronic cigarettes
that the Customs Administration has submitted to test since
2015.
Figure 5-3 The appearance, diagram and detection rate of the e-cigarette
B. In the inspection of instant coffee packs submitted by Taipei City Police
Department, it reveals that the samples contain not only the Schedule III drug
Ketamine, but a suspicious un-identified component was also discovered. Through
GC-MS analysis and compared the results to the Analytic Laboratory Urine
and Drug Abuse Report System (UDARS), the component has been confirmed
as synthetic 6-methoxy Methylone (Figure 5-4) which is inspected for the first
time in Taiwan. The activity and toxicity of 6-methoxy Methylone still remain
unknown at present; the misuse of such component may result in sever impacts
on public health. TFDA announced the news promptly on October 12 to warn
compatriots to be aware of it. Owing to the components identified as the analogue
of Methylone and Mephedrone, it is speculated to act similar pharmacological
properties as the Schedule II drugs Mephedrone and MDMA. In view of above,
TFDA suggests Narcotics Review Committee, Ministry of Justice to include the
component as narcotics to boost the strength of defense for anti-drug ties.
C. The botulinum toxin preparations submitted by Custom Administration, Ministry
of Finance in March, 2016 were found without containing botulinum toxin type
A. Because the appearance of the product is similar to the ones that have been
approved, this is the first adulteration of botulinum toxin preparations found
in Taiwan (Figure 5-5). TFDA issued the news immediately to remind fellow
citizens. After being disclosed by social media, Taiwanese Dermatological
Association and Consumers' Foundation, Chinese Taipei both held press
conferences to educate the public about how to identify adulterate medicinal
products to prevent fraud.
68