Page 63 - Taiwan Food and Drug Administration 2016 Annual Report
P. 63
2016 ANNUAL
REPORT
2. Pre-Market Approval & Registration of Cosmetics and Cosmetic Advertisement Examination
(1) Inspection and registration of medicated cosmetics
a. In 2015, a total of 1,822 applications for medicated cosmetics were received, of which a total
of 1,558 were approved (Table 2-5-1).
Table2-5-1 Number of licenses approved for medicated cosmetics from 2010 to 2015
Year Total applications License approved Approval rate (%)
2010 1,594 1,437 90.2 Part II - Key Administrative Results Cosmetics Management
2011 1,634 1,519 93.0
2012 1,721 1,482 86.1
2013 1,650 1,506 91.3
2014 1,900 1,661 87.4
2015 1,822 1,558 85.5
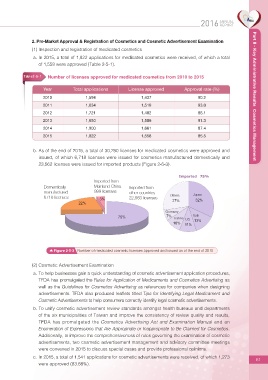
b. As of the end of 2015, a total of 30,280 licenses for medicated cosmetics were approved and
issued, of which 6,718 licenses were issued for cosmetics manufactured domestically and
23,562 licenses were issued for imported products (Figure 2-5-3).
Imported 75%
Imported from
Domestically Mainland China Imported from
manufactured 999 licenses other countries Japan
6718 licenses 3% 22,563 licenses Others
22% 27% 32%
Germany
75% 7% France US Italy
10% 11% 13%
Figure 2-5-3 Number of medicated cosmetic licenses approved and issued as of the end of 2015
(2) Cosmetic Advertisement Examination
a. To help businesses gain a quick understanding of cosmetic advertisement application procedures,
TFDA has promulgated the Rules for Application of Medicaments and Cosmetics Advertising as
well as the Guidelines for Cosmetics Advertising as references for companies when designing
advertisements. TFDA also produced leaflets titled Tips for Identifying Legal Medicament and
Cosmetic Advertisements to help consumers correctly identify legal cosmetic advertisements.
b. To unify cosmetic advertisement review standards amongst health bureaus and departments
of the six municipalities of Taiwan and improve the consistency of review quality and results,
TFDA has promulgated the Cosmetics Advertising Act and Examination Manual and an
Enumeration of Expressions that Are Appropriate or Inappropriate to Be Claimed for Cosmetics.
Additionally, to improve the comprehensiveness of rules governing the examination of cosmetic
advertisements, two cosmetic advertisement management and advisory committee meetings
were convened in 2015 to discuss special cases and provide professional opinions.
c. In 2015, a total of 1,541 applications for cosmetic advertisements were received, of which 1,273
61
were approved (83.88%).