Page 66 - Taiwan Food and Drug Administration 2016 Annual Report
P. 66
Taiwan Food and Drug Adminstration
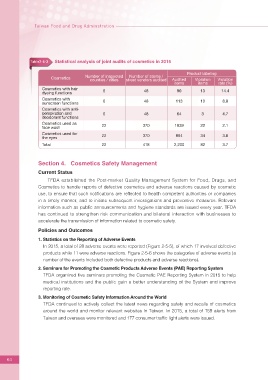
Table2-5-3 Statistical analysis of joint audits of cosmetics in 2015
Product labeling
Number of inspected Number of stores /
Cosmetics
counties / cities street vendors audited Audited Violation Violation
items items rate (%)
Cosmetics with hair 6 48 90 13 14.4
dyeing functions
Cosmetics with 6 48 113 10 8.8
sunscreen functions
Cosmetics with anti-
perspiration and 6 48 64 3 4.7
deodorant functions
Cosmetics used as
face wash 22 370 1039 22 2.1
Cosmetics used for
the eyes 22 370 894 34 3.8
Total 22 418 2,200 82 3.7
Section 4. Cosmetics Safety Management
Current Status
TFDA established the Post-market Quality Management System for Food, Drugs, and
Cosmetics to handle reports of defective cosmetics and adverse reactions caused by cosmetic
use, to ensure that such noti?cations are re?ected to health competent authorities or companies
in a timely manner, and to initiate subsequent investigations and preventive measures. Relevant
information such as public announcements and hygiene standards are issued every year. TFDA
has continued to strengthen risk communication and bilateral interaction with businesses to
accelerate the transmission of information related to cosmetic safety.
Policies and Outcomes
1. Statistics on the Reporting of Adverse Events
In 2015, a total of 28 adverse events were reported (Figure 2-5-5), of which 17 involved defective
products while 11 were adverse reactions. Figure 2-5-6 shows the categories of adverse events (a
number of the events included both defective products and adverse reactions).
2. Seminars for Promoting the Cosmetic Products Adverse Events (PAE) Reporting System
TFDA organized five seminars promoting the Cosmetic PAE Reporting System in 2015 to help
medical institutions and the public gain a better understanding of the System and improve
reporting rate.
3. Monitoring of Cosmetic Safety Information Around the World
TFDA continued to actively collect the latest news regarding safety and recalls of cosmetics
around the world and monitor relevant websites in Taiwan. In 2015, a total of 159 alerts from
Taiwan and overseas were monitored and 177 consumer traf?c light alerts were issued.
64