Page 64 - Taiwan Food and Drug Administration 2016 Annual Report
P. 64
Taiwan Food and Drug Adminstration
Section 2. Cosmetics Source Management
Current Status
Cosmetics manufacturers are currently required to submit documented information to the
Industrial Development Bureau (IDB) of the Ministry of Economic Affairs (MOEA). The IDB then
assembles an audit team to perform the audit. Manufacturers that pass the audit may then apply for
a GMP certi?cate from TFDA. As management after pre-market registration will be exempted in the
future, TFDA is planning to introduce a new section on the Cosmetic Product Noti?cation System in
the revised Statute for Control of Cosmetic Hygiene. Prior to these legal revisions, business owners
are encouraged to voluntarily register themselves at the Cosmetic Product Notification Portal.
Once legal revisions are approved and enforced, business registration at the portal will become
mandatory in order to achieve the purposes of effective management and consumer protection.
Policies and Outcomes
1. Production Source Control
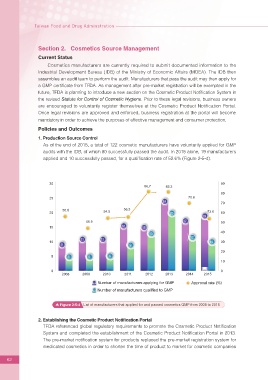
As of the end of 2015, a total of 122 cosmetic manufacturers have voluntarily applied for GMP
audits with the IDB, of which 80 successfully passed the audit. In 2015 alone, 19 manufacturers
applied and 10 successfully passed, for a quali?cation rate of 52.6% (Figure 2-5-4).
30 90
86.7 83.3
80
25 70.6
24 70
55.6 56.3
20 54.5 20 52.6 60
19
45.5 17 50
15 16 15
13 40
11 11 12
10 10 30
9 9
20
5 5 5 6
10
0 0
2008 2009 2010 2011 2012 2013 2014 2015
Number of manufacturers applying for GMP Approval rate (%)
Number of manufacturers quali?ed to GMP
Figure 2-5-4 List of manufacturers that applied for and passed cosmetics GMP from 2008 to 2015
2. Establishing the Cosmetic Product Notification Portal
TFDA referenced global regulatory requirements to promote the Cosmetic Product Noti?cation
System and completed the establishment of the Cosmetic Product Noti?cation Portal in 2013.
The pre-market noti?cation system for products replaced the pre-market registration system for
medicated cosmetics in order to shorten the time of product to market for cosmetic companies
62