Page 36 - Taiwan Food and Drug Administration 2016 Annual Report
P. 36
Taiwan Food and Drug Adminstration
Section 2. Medicinal Products Source Management
Current status
To improve manufacturing quality of medicinal products and align to international standards,
Taiwan began enforcing increasingly stringent laws and international harmonization for the
management of medicinal product factories. In 1982, Taiwan began implementing Good
Manufacturing Practice (GMP) for pharmaceuticals and enforced the current Good Manufacturing
Practice (cGMP) in 1995. In 2007, Taiwan began promoting GMP standards of the Pharmaceutical
Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S GMP), and
attained total enforcement by the end of 2014, achieving complete harmonization of Taiwan's
pharmaceutical manufacturing standards with international standards. To strengthen production
quality management, Taiwan promulgated GMP standards for active pharmaceutical ingredients
(APIs) in 2013 and achieved complete enforcement by the end of 2015. The GMP for Modern
Pharmaceutical Products (Part 3) was released in 2015 with complete enforcement scheduled by
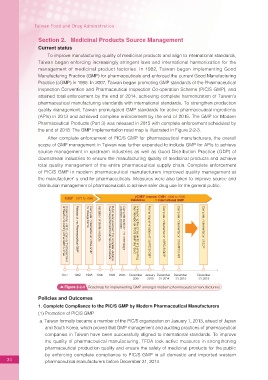
the end of 2018. The GMP implementation road map is illustrated in Figure 2-2-3.
After complete enforcement of PIC/S GMP for pharmaceutical manufacturers, the overall
scope of GMP management in Taiwan was further expanded to include GMP for APIs to achieve
source management in upstream industries as well as Good Distribution Practice (GDP) of
downstream industries to ensure the manufacturing quality of medicinal products and achieve
total quality management of the entire pharmaceutical supply chain. Complete enforcement
of PIC/S GMP in modern pharmaceutical manufacturers improved quality management at
the manufacturer' s end for pharmaceuticals. Measures were also taken to improve source and
distribution management of pharmaceuticals to achieve safer drug use for the general public.
GMP 1977 to 1988 cGMP (current GMP) 1995 to 2005
Validation Formal implementation of the PIC/S GMP International GMP Complete implementation of GDP
for dosage forms
validation process
Validation of sterile products
Complete establish the three-stage
Release of the Pharmaceutical GMP
the international PIC/S GMP standard
Complete implementation of the GMP
of Health and drafting of the GMP outlines
Complete implementation of PIC/S GMP
Complete implementation of GMP for API
must be performed for all medicinal products
Announcing the schedule for implementing
Public announcement that complete validation
Observational visit to Japan by the Department
1977 1982 1988 1995 1999 2005 December January December December December
2007 2010 31 2014 31 2015 31 2018
Figure 2-2-3 Roadmap for implementing GMP amongst modern pharmaceutical manufacturers
Policies and Outcomes
1. Complete Compliance to the PIC/S GMP by Modern Pharmaceutical Manufacturers
(1) Promotion of PIC/S GMP
a. Taiwan formally became a member of the PIC/S organization on January 1, 2013, ahead of Japan
and South Korea, which proved that GMP management and auditing practices of pharmaceutical
companies in Taiwan have been successfully aligned to international standards. To improve
the quality of pharmaceutical manufacturing, TFDA took active measures in strengthening
pharmaceutical production quality and ensure the safety of medicinal products for the public
by enforcing complete compliance to PIC/S GMP in all domestic and imported western
34 pharmaceutical manufacturers before December 31, 2014.