Page 34 - Taiwan Food and Drug Administration 2016 Annual Report
P. 34
Taiwan Food and Drug Adminstration
Policies and Outcomes
1. Comprehensive Regulations and Standards
A number of key addenda and revisions were made to medicinal product management laws and
related standards in 2015. Revisions include the following: Pharmaceutical Affairs Act and the
Rare Disease and Orphan Drug Act, Regulation of Bioavailability and Bioequivalence Studies,
regulations for applying for drug hazard relief, and regulations for registration of medicinal
products. Promulgated laws include the following: Regulations for Medicament Recall, standards
for the registration of monoclonal antibodies for biosimilars, standards for determining the
adequacy of donors of cell therapy products, and standards for the inspection, registration,
and review of medicinal products. TFDA also announced the need to include the composition
or name of the excipient upon the package inserts of the medicinal products and that the active
pharmaceutical ingredient (API) used within the preparation must be compliant with Good
Manufacturing Practice (GMP) for pharmaceuticals. Refer to Table 8 of Annex I for details on the
revisions as well as promulgation of new laws.
2. Medicinal Product Registration Management
Medicinal product registration can be divided into active pharmaceutical ingredients (API) and
its preparations, the latter of which can be further divided into new drugs, bioagents, generic
drugs, and orphan drugs. Where local clinical trials or bioavailability (BA) and bioequivalence (BE)
study results must be provided as attachments for inspection and registration applications, the
corresponding project plans and reports must be reviewed and approved as well. The number of
drug permit licenses approved by TFDA every year is listed in Table 9 of Annex I.
(1) Pre-market Inspection and Registration for Medicinal Products
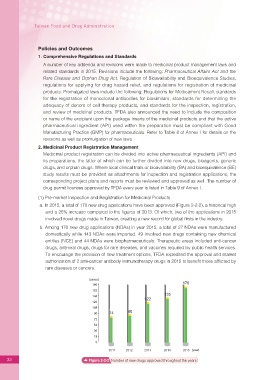
a. In 2015, a total of 170 new drug applications have been approved (Figure 2-2-2), a historical high
and a 26% increase compared to the ?gures of 2013. Of which, two of the applications in 2015
involved novel drugs made in Taiwan, creating a new record for global ?rsts in the industry.
b. Among 170 new drug applications (NDAs) in year 2015, a total of 27 NDAs were manufactured
domestically while 143 NDAs were imported. 49 involved new drugs containing new chemical
entities (NCE) and 44 NDAs were biopharmaceuticals. Therapeutic areas included anti-cancer
drugs, anti-viral drugs, drugs for rare diseases, and vaccines required by public health services.
To encourage the provision of new treatment options, TFDA expedited the approval and market
authorization of 2 anti-cancer antibody immunotherapy drugs in 2015 to bene?t those af?icted by
rare diseases or cancers.
(cases)
180 170
162
144 135
122
126
108
90 74 80
72
54
36
18
0
2011 2012 2013 2014 2015 (year)
32 Figure 2-2-2 Number of new drugs approved throughout the years