Page 24 - Taiwan Food and Drug Administration 2016 Annual Report
P. 24
Taiwan Food and Drug Adminstration
out compliance audits (Table 2-1-3), maximizing the effectiveness of limited resources for food
factory supervision and management efforts. TFDA also rated food factories according to the
results of factory audits, using these data as a reference for planning factory inspection policies
while encouraging food businesses to place greater importance on consumer food safety and
enforce proper controls over sanitation and safety.
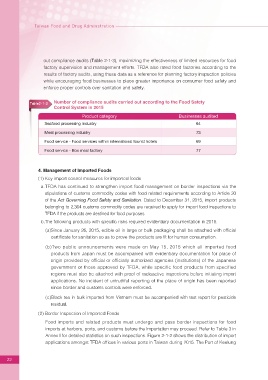
Table2-1-3 Number of compliance audits carried out according to the Food Safety
Control System in 2015
Product category Businesses audited
Seafood processing industry 64
Meat processing industry 73
Food service - Food services within international tourist hotels 69
Food service - Box meal factory 77
4. Management of Imported Foods
(1) Key import control measures for imported foods
a.TFDA has continued to strengthen import food management on border inspections via the
stipulations of customs commodity codes with food related requirements according to Article 30
of the Act Governing Food Safety and Sanitation. Dated to December 31, 2015, import products
belonging to 2,364 customs commodity codes are required to apply for import food inspections to
TFDA if the products are destined for food purposes.
b.The following products with speci?c risks required evidentiary documentation in 2015.
(a)Since January 26, 2015, edible oil in large or bulk packaging shall be attached with of?cial
certi?cate for sanitation so as to prove the products are ?t for human consumption.
(b)Two public announcements were made on May 15, 2015 which all imported food
products from Japan must be accompanied with evidentiary documentation for place of
origin provided by official or officially authorized agencies (institutions) of the Japanese
government or those approved by TFDA, while specific food products from specified
regions must also be attached with proof of radioactive inspections before initiating import
applications. No incident of untruthful reporting of the place of origin has been reported
since border and customs controls were enforced.
(c)Black tea in bulk imported from Vietnam must be accompanied with test report for pesticide
residual.
(2) Border Inspection of Imported Foods
Food imports and related products must undergo and pass border inspections for food
imports at harbors, ports, and customs before the importation may proceed. Refer to Table 3 in
Annex I for detailed statistics on such inspections. Figure 2-1-2 shows the distribution of import
applications amongst TFDA of?ces in various ports in Taiwan during 2015. The Port of Keelung
22