Page 23 - Taiwan Food and Drug Administration 2016 Annual Report
P. 23
學名藥21,478張,81%
2016
(國產19,162 ; 輸入2,316張) ANNUAL
REPORT
新藥1,774張,6.7%
(國產495 ; 輸入1,279張) 原料藥2,610張,9.9%
(國產600 ; 輸入2,010張)
and their factories / plants, 10,000 food importers, over 700 food utensil containers and packaging
生物製劑373張,1.4%
罕藥58張,0.2% (國產14 ; 輸入359張)
businesses, over 300 food additive businesses, and over 77 food cleanser businesses. TFDA shall
(國產13張 ; 輸入45張)
continue to strive towards registering every food-related business in Taiwan.
(2) Consumers and food businesses can both access the Food and Medicinal Business
Registration Platform (http://fadenbook.fda.gov.tw/) to check information on food business Part II - Key Administrative Results Food Management
registration and quickly acquire the latest announcements and information. The system also
made the government more effectively control over the distribution of food businesses and
improve management ef?ciency.
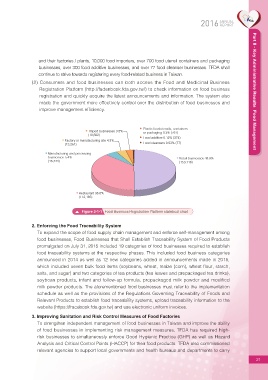
Plastic food utensils, containers
Import businesses 3.5% or packaging 0.3% (767)
(10,602)
Food additive 0.12% (374)
Factory or manufacturing site 4.3%
(13,261) Food cleansers 0.03% (77)
Manufacturing and processing
Number of registration licenses approved and issued for specific businesses 5.4% Retail businesses 49.9%
food products as of the end of 2015 (16,444) (153,116)
Restaurant 36.6%
(112,165)
Figure 2-1-1 Food Business Registration Platform statistical chart
2. Enforcing the Food Traceability System
To expand the scope of food supply chain management and enforce self-management among
food businesses, Food Businesses that Shall Establish Traceability System of Food Products
promulgated on July 31, 2015 included 19 categories of food businesses required to establish
food traceability systems at the respective phases. This included food business categories
announced in 2014 as well as 12 new categories added in announcements made in 2015,
which included seven bulk food items (soybeans, wheat, maize (corn), wheat flour, starch,
salts, and sugar) and two categories of tea products (tea leaves and prepackaged tea drinks),
soybean products, infant and follow-up formula, prepackaged milk powder and modified
milk powder products. The aforementioned food businesses must refer to the implementation
schedule as well as the provisions of the Regulations Governing Traceability of Foods and
Relevant Products to establish food traceability systems, upload traceability information to the
website (https://fracebook.fda.gov.tw) and use electronic uniform invoices.
3. Improving Sanitation and Risk Control Measures of Food Factories
To strengthen independent management of food businesses in Taiwan and improve the ability
of food businesses in implementing risk management measures, TFDA has required high-
risk businesses to simultaneously enforce Good Hygienic Practice (GHP) as well as Hazard
Analysis and Critical Control Points (HACCP) for their food products. TFDA also commissioned
relevant agencies to support local governments and health bureaus and departments to carry
21