Page 22 - Taiwan Food and Drug Administration 2016 Annual Report
P. 22
Taiwan Food and Drug Adminstration
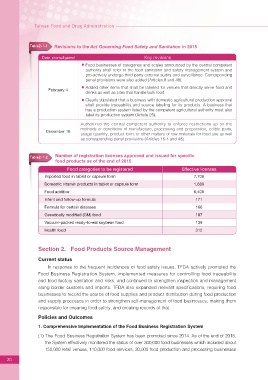
Table2-1-1 Revisions to the Act Governing Food Safety and Sanitation in 2015
Date promulgated Key revisions
Food businesses of categories and scales announced by the central competent
authority shall refer to the food sanitation and safety management system and
pro-actively undergo third party external audits and surveillance. Corresponding
penal provisions were also added (Articles 8 and 48).
Added other items that shall be labeled for venues that directly serve food and
February 4
drinks as well as sites that handle bulk food.
Clearly stipulated that a business with domestic agricultural production approval
shall provide traceability and source labeling for its products. A business that
has a production system listed by the competent agricultural authority must also
label its production system (Article 25).
Authorized the central competent authority to enforce restrictions up on the
methods or conditions of manufacture, processing and preparation, edible parts,
December 16
usage quantity, product form, or other matters of raw materials for food use as well
as corresponding penal provisions (Articles 15-1 and 48).
Table2-1-2 Number of registration licenses approved and issued for specific
food products as of the end of 2015
Food categories to be registered Effective licenses
Imported food in tablet or capsule form 7,706
Domestic vitamin products in tablet or capsule form 1,689
Food additive 6,406
Infant and follow-up formula 171
Formula for certain diseases 166
Genetically modified (GM) food 107
Vacuum-packed ready-to-eat soybean food 139
Health food 312
Section 2. Food Products Source Management
Current status
In response to the frequent incidences of food safety issues, TFDA actively promoted the
Food Business Registration System, implemented measures for controlling food traceability
and food factory sanitation and risks, and continued to strengthen inspection and management
along border customs and imports. TFDA also expanded relevant speci?cations, requiring food
businesses to record the source of food supplies and product distribution during food production
and supply processes in order to strengthen self-management of food businesses, making them
responsible for ensuring food safety, and creating records of that.
Policies and Outcomes
1. Comprehensive Implementation of the Food Business Registration System
(1) The Food Business Registration System has been promoted since 2014. As of the end of 2015,
the System effectively monitored the status of over 300,000 food businesses which included about
150,000 retail venues, 110,000 food services, 30,000 food production and processing businesses
20