
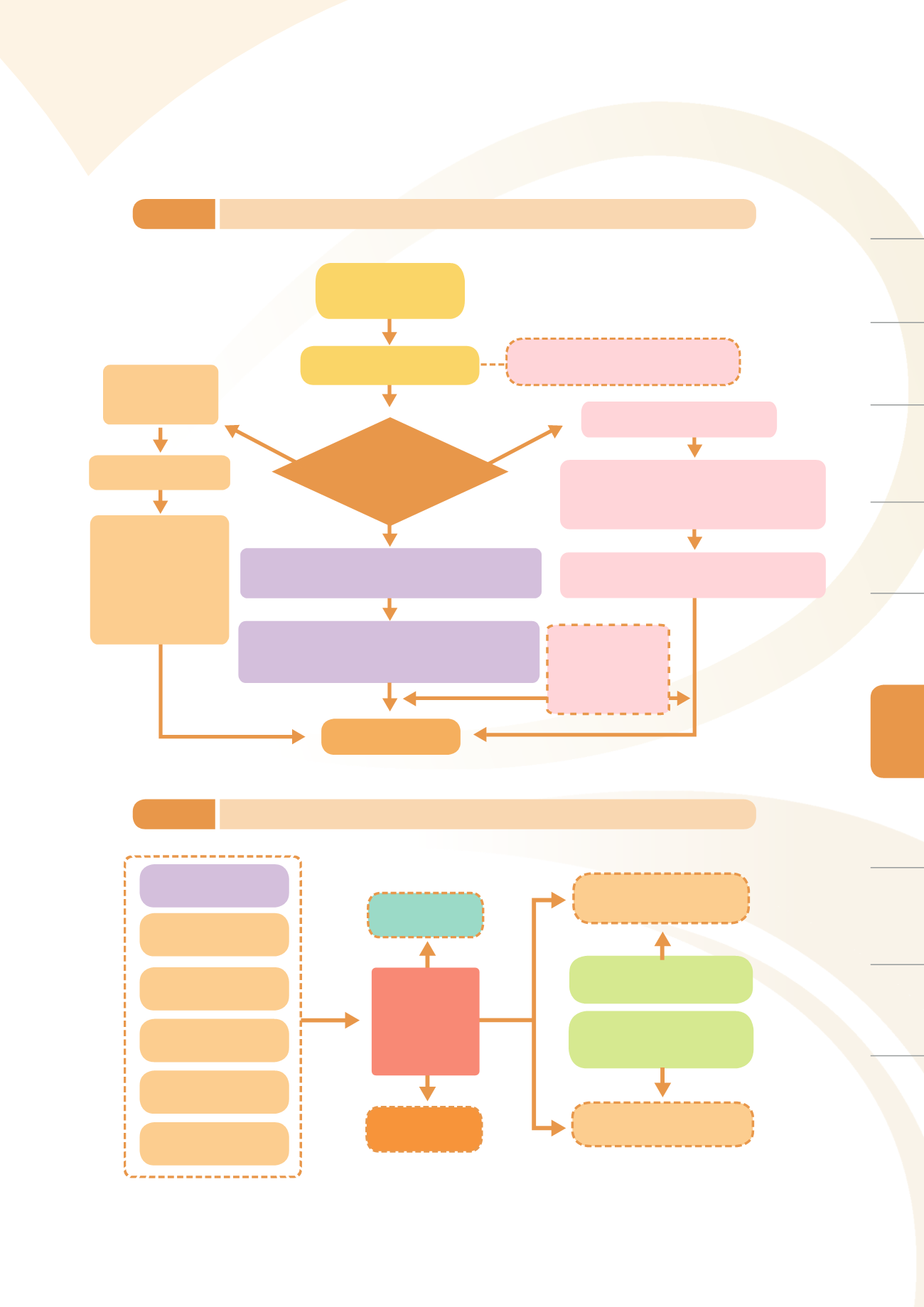
Hold the ERT meeting, Generate a name list of respon-
sible and supporting personnel and delegation of tasks,
Activiate the emergency response operation mecha-
nism, Carry out tasks as delegated by the ERT
The ’responsible owner’
must review the
handling of the entire
incident from the very
beginning and generate
a report for future
reference and improve-
ment.
Independently handled
by the responsible owner
Level 2
(cross-departmental
hierarchy)
Level 3
(Group level)
Level 1
(TFDA level)
Responsible
Owner Determine the
degree of impact of the
incident Categorize
the incident
review the incident
once it dies down
3 groups, offices, or centers must
work together to solve the issue
The Deputy Director-General or Chief Secretary
must supervise and report incident handling
updates to various units
Responsible units
may independently
handle to deescalate
the incident
When the incident
has been somewhat
alleviated, reduce
the incident level or
disband the ERT
After being evaluated by the responsible owner or
the risk monitoring center, the incident will then be
submitted to the Director-General or Deputy
Director-General for approval before convening a
work coordination meeting
Emergency incident
(major public opinion
or major incident)
Relevant group / office / center
Receiving emergency news
Each unit involved takes action based on
the Guidelines
for Handling and Reporting
Major Public Opinion and Incidents.
Complete and highest emergency response command
level Report the incident to TFDA director-general to
establish an Emergency Response Team (ERT)
Figure 7-1
Emergency incident handling workflow of the Food and Drugs Administration
Figure 7-2
Risk control workflow
2015 Annual Report
73
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
Sources of risk
information
Risk assessment and
Risk management
Post-market surveillance
of the products
Emergency
response
Risk
warning
Risk
Monitoring
Task Force
Food safety
Risk Assessment
Consultation Committee
Post-market Surveil-
lance Plan Review
Committee
Domestics
public opinion
Global product
surveillance information
Global product
safety information
Risk assessment
information
Domestic product
surveillance
information



















