Page 114 - 2023 Taiwan Food and Drug Administration Annual Report
P. 114
2023 Taiwan Food and
Drug Administration
Annual Report
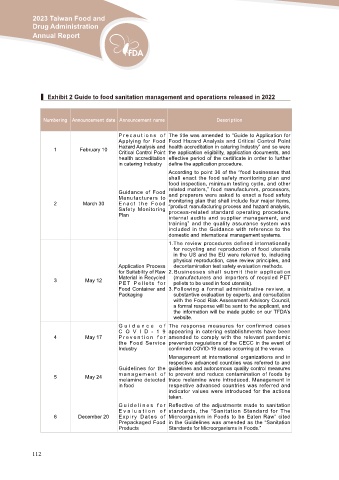
▍ Exhibit 2 Guide to food sanitation management and operations released in 2022
Numbering Announcement date Announcement name Description
P r e c a u t i o n s o f The title was amended to “Guide to Application for
Applying for Food Food Hazard Analysis and Critical Control Point
1 February 10 Hazard Analysis and health accreditation in catering Industry” and so were
Critical Control Point the application eligibility, application documents, and
health accreditation effective period of the certificate in order to further
in catering Industry define the application procedure.
According to point 36 of the “food businesses that
shall enact the food safety monitoring plan and
food inspection, minimum testing cycle, and other
2 March 30 Guidance of Food related matters,” food manufacturers, processors,
Manufacturers to and preparers were asked to enact a food safety
Enact the Food monitoring plan that shall include four major items,
Safety Monitoring “product manufacturing process and hazard analysis,
Plan process-related standard operating procedure,
internal audits and supplier management, and
training” and the quality assurance system was
included in the Guidance with reference to the
domestic and international management systems.
1.The review procedures defined internationally
for recycling and reproduction of food utensils
in the US and the EU were referred to, including
physical reproduction, case review principles, and
Application Process decontamination test safety evaluation methods.
for Suitability of Raw 2 . B u s i n e s s e s s h a l l s u b m i t t h e i r a p p l i c a t i o n
3 May 12 Material in Recycled (manufacturers and importers of recycled PET
P E T P e l l e t s f o r pellets to be used in food utensils).
Food Container and 3.Following a formal administrative review, a
Packaging substantive evaluation by experts, and consultation
with the Food Risk Assessment Advisory Council,
a formal response will be sent to the applicant, and
the information will be made public on our TFDA’s
website.
G u i d a n c e o f The response measures for confirmed cases
C O V I D - 1 9 appearing in catering establishments have been
4 May 17 P r e v e n t i o n f o r amended to comply with the relevant pandemic
the Food Service prevention regulations of the CECC in the event of
Industry confirmed COVID-19 cases occurring at the venue.
Management at international organizations and in
respective advanced countries was referred to and
Guidelines for the guidelines and autonomous quality control measures
5 May 24 m a n a g e m e n t o f to prevent and reduce contamination of foods by
melamine detected trace melamine were introduced. Management in
in food respective advanced countries was referred and
indicator values were introduced for the actions
taken.
G u i d e l i n e s f o r Reflective of the adjustments made to sanitation
E v a l u a t i o n o f standards, the “Sanitation Standard for The
6 December 20 E x p i r y D a t e s o f Microorganism in Foods to be Eaten Raw” cited
Prepackaged Food in the Guidelines was amended as the “Sanitation
Products Standards for Microorganisms in Foods.”
112