Page 110 - 2023 Taiwan Food and Drug Administration Annual Report
P. 110
2023 Taiwan Food and
Drug Administration
Annual Report
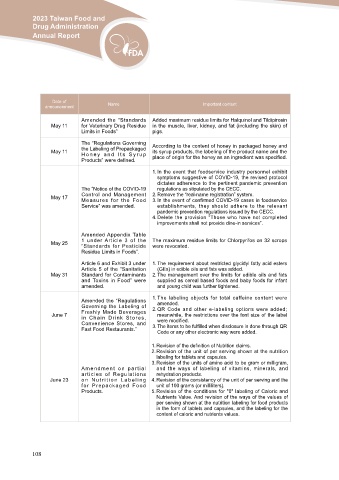
Date of Name Important content
announcement
Amended the “Standards Added maximum residue limits for Halquinol and Tildipirosin
May 11 for Veterinary Drug Residue in the muscle, liver, kidney, and fat (including the skin) of
May 11
Limits in Foods” pigs.
May 17
The “Regulations Governing According to the content of honey in packaged honey and
May 25 the Labeling of Prepackaged its syrup products, the labeling of the product name and the
May 31 Honey and Its Syrup place of origin for the honey as an ingredient was specified.
June 7 Products” were defined.
The “Notice of the COVID-19 1. In the event that foodservice industry personnel exhibit
Control and Management symptoms suggestive of COVID-19, the revised protocol
Measures for the Food dictates adherence to the pertinent pandemic prevention
Service” was amended. regulations as stipulated by the CECC.
2. Remove the “real-name registration” system.
3. In the event of confirmed COVID-19 cases in foodservice
establishments, they should adhere to the relevant
pandemic prevention regulations issued by the CECC.
4. Delete the provision “Those who have not completed
improvements shall not provide dine-in services”.
Amended Appendix Table The maximum residue limits for Chlorpyrifos on 32 scrops
1 under Article 3 of the were revocated.
“Standards for Pesticide
Residue Limits in Foods”.
Article 6 and Exhibit 3 under 1. The requirement about restricted glycidyl fatty acid esters
Article 5 of the “Sanitation (GEs) in edible oils and fats was added.
Standard for Contaminants
and Toxins in Food” were 2. The management over the limits for edible oils and fats
amended. supplied as cereal based foods and baby foods for infant
and young child was further tightened.
Amended the “Regulations 1. The labeling objects for total caffeine content were
Governing the Labeling of amended.
Freshly Made Beverages
in Chain Drink Stores, 2. QR Code and other e-labeling options were added;
Convenience Stores, and meanwhile, the restrictions over the font size of the label
Fast Food Restaurants.” were modified.
3. The items to be fulfilled when disclosure is done through QR
Code or any other electronic way were added.
June 23 Amendment on partial 1. Revision of the definition of Nutrition claims.
articles of Regulations 2. Revision of the unit of per serving shown at the nutrition
on Nutrition Labeling
for Prepackaged Food labeling for tablets and capsules.
Products. 3. Revision of the units of amino acid to be gram or milligram,
and the ways of labeling of vitamins, minerals, and
rehydration products.
4. Revision of the consistency of the unit of per serving and the
unit of 100 grams (or milliliters).
5. Revision of the conditions for "0" labeling of Caloric and
Nutrients Value. And revision of the ways of the values of
per serving shown at the nutrition labeling for food products
in the form of tablets and capsules, and the labeling for the
content of caloric and nutrients values.
108