Page 108 - 2023 Taiwan Food and Drug Administration Annual Report
P. 108
2023 Taiwan Food and
Drug Administration
Annual Report
Appendix 2. Important 2022 achievements and statistics
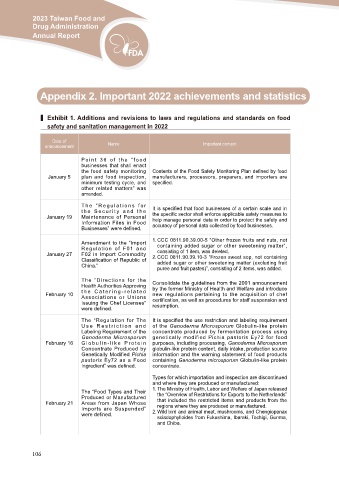
▍ Exhibit 1. Additions and revisions to laws and regulations and standards on food
safety and sanitation management in 2022
Date of Name Important content
announcement
January 5 Point 36 of the “food Contents of the Food Safety Monitoring Plan defined by food
January 19 businesses that shall enact manufacturers, processors, preparers, and importers are
January 27 the food safety monitoring specified.
February 10 plan and food inspection,
February 16 minimum testing cycle, and
other related matters” was
February 21 amended.
The “Regulations for It is specified that food businesses of a certain scale and in
the Security and the the specific sector shall enforce applicable safety measures to
Maintenance of Personal help manage personal data in order to protect the safety and
Information Files in Food accuracy of personal data collected by food businesses.
Businesses” were defined.
Amendment to the “Import 1. CCC 0811.90.39.00-5 “Other frozen fruits and nuts, not
Regulation of F01 and containing added sugar or other sweetening matter”,
F02 in Import Commodity consisting of 1 item, was deleted.
Classification of Republic of
China.” 2. CCC 0811.90.39.10-3 “Frozen sweet sop, not containing
added sugar or other sweetening matter (excluding fruit
puree and fruit pastes)”, consisting of 2 items, was added.
The “Directions for the Consolidate the guidelines from the 2001 announcement
Health Authorities Approving by the former Ministry of Health and Welfare and introduce
the Catering-related new regulations pertaining to the acquisition of chef
Associations or Unions certification, as well as procedures for staff suspension and
Issuing the Chef Licenses” resumption.
were defined.
The “Regulation for The It is specified the use restriction and labeling requirement
Use Restriction and of the Ganoderma Microsporum Globulin-like protein
Labeling Requirement of the concentrate produced by fermentation process using
Ganoderma Microsporum genetically modified Pichia pastoris Ey72 for food
Globulin-like Protein purposes, including processing, Ganoderma Microsporum
Concentrate Produced by globulin-like protein content, daily intake, production source
Genetically Modified Pichia information and the warning statement of food products
pastoris Ey72 as a Food containing Ganoderma microsporum Globulin-like protein
Ingredient” was defined. concentrate.
The “Food Types and Their Types for which importation and inspection are discontinued
Produced or Manufactured and where they are produced or manufactured:
Areas from Japan Whose 1. The Ministry of Health, Labor and Welfare of Japan released
Imports are Suspended”
were defined. the “Overview of Restrictions for Exports to the Netherlands”
that included the restricted items and products from the
regions where they are produced or manufactured.
2. Wild bird and animal meat, mushrooms, and Chengiopanax
sciadophylloides from Fukushima, Ibaraki, Tochigi, Gunma,
and Chiba.
106