Page 115 - 2020Taiwan Food and Drug Administration Annual Report
P. 115
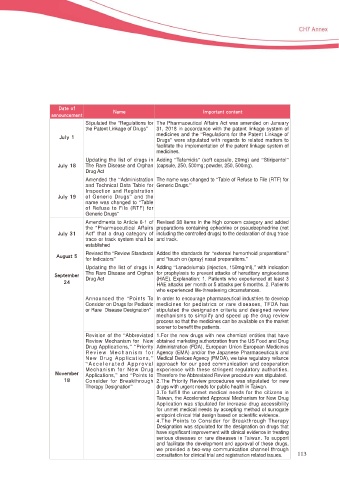
%BUF PG
/BNF *NQPSUBOU DPOUFOU
BOOPVODFNFOU
Stipulated the “Regulations for The Pharmaceutical Affairs Act was amended on January
the Patent Linkage of Drugs” 31, 2018 in accordance with the patent linkage system of
medicines and the “Regulations for the Patent Linkage of
+VMZ
Drugs” were stipulated with regards to related matters to
facilitate the implementation of the patent linkage system of
medicines.
Updating the list of drugs in Adding “Tafamidis” (soft capsule, 20mg) and “Stiripentol”
+VMZ The Rare Disease and Orphan (capsule, 250, 500mg; powder, 250, 500mg).
Drug Act
Amended the “Administration The name was changed to “Table of Refuse to File (RTF) for
and Technical Data Table for Generic Drugs.”
Inspection and Registration
+VMZ of Generic Drugs” and the
name was changed to “Table
of Refuse to File (RTF) for
Generic Drugs”
Amendments to Article 6-1 of Revised 38 items in the high concern category and added
the "Pharmaceutical Affairs preparations containing ephedrine or pseudoephedrine (not
+VMZ Act" that a drug category of including the controlled drugs) to the declaration of drug trace
trace or track system shall be and track.
established
Revised the “Review Standards Added the standards for “H[WHUQDO KHPRUUKRLG SUHSDUDWLRQV”
"VHVTU for Indicators” and “touch on (spray) nasal preparations.”
Updating the list of drugs in Adding “Lanadelumab (injection, 150mg/ml),” with indication
The Rare Disease and Orphan IRU SURSK\OD[LV WR SUHYHQW DWWDFNV RI KHUHGLWDU\ DQJLRHGHPD
4FQUFNCFS Drug Act +$( ([SODQDWLRQ 3DWLHQWV ZKR H[SHULHQFHG DW OHDVW
HAE attacks per month or 5 attacks per 6 months. 2. Patients
ZKR H[SHULHQFHG OLIH WKUHDWHQLQJ FLUFXPVWDQFHV
Announced the “Points To In order to encourage pharmaceutical industries to develop
Consider on Drugs for Pediatric medicines for pediatrics or rare diseases, TFDA has
or Rare Disease Designation” stipulated the designation criteria and designed review
mechanisms to simplify and speed up the drug review
process so that the medicines can be available on the market
VRRQHU WR EHQH¿W WKH SDWLHQWV
Revision of the “Abbreviated 1.For the new drugs with new chemical entities that have
Review Mechanism for New REWDLQHG PDUNHWLQJ DXWKRUL]DWLRQ IURP WKH 86 )RRG DQG 'UXJ
Drug Applications,” “Priority Administration (FDA), European Union European Medicines
Review Mechanism for Agency (EMA) and/or the Japanese Pharmaceuticals and
New Drug Applications,” Medical Devices Agency (PMDA), we take regulatory reliance
“ Accelerated Approval approach for our good communication and cooperation
Mechanism for New Drug H[SHULHQFH ZLWK WKHVH VWULQJHQW UHJXODWRU\ DXWKRULWLHV
/PWFNCFS Applications,” and “Points to Therefore the Abbreviated Review procedure was stipulated.
Consider for Breakthrough 2.The Priority Review procedures was stipulated for new
Therapy Designation” drugs with urgent needs for public health in Taiwan.
7R IXOILOO WKH XQPHW PHGLFDO QHHGV IRU WKH FLWL]HQV LQ
Taiwan, the Accelerated Approval Mechanism for New Drug
Application was stipulated for increase drug accessibility
for unmet medical needs by accepting method of surrogate
HQGSRLQW FOLQLFDO WULDO GHVLJQ EDVHG RQ VFLHQWL¿F HYLGHQFH
4.The Points to Consider for Breakthrough Therapy
Designation was stipulated for the designation on drugs that
KDYH VLJQL¿FDQW LPSURYHPHQW ZLWK FOLQLFDO HYLGHQFH LQ WUHDWLQJ
serious diseases or rare diseases in Taiwan. To support
and facilitate the development and approval of these drugs,
we provided a two-way communication channel through
consultation for clinical trial and registration related issues. 113