Page 45 - 2017食品藥物管理署年報(英文版)
P. 45
2017 Taiwan Food and Drug Administration Annual Report Chapter 3. Source and Manufacturing Management
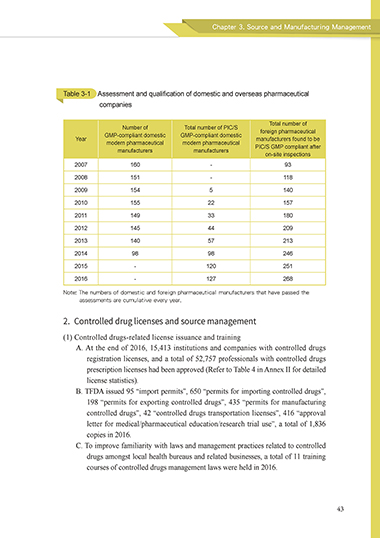
Table 3-1 Assessment and qualification of domestic and overseas pharmaceutical
companies
Total number of
Number of Total number of PIC/S
GMP-compliant domestic GMP-compliant domestic foreign pharmaceutical
Year manufacturers found to be
modern pharmaceutical modern pharmaceutical PIC/S GMP compliant after
manufacturers manufacturers
on-site inspections
2007 160 - 93
2008 151 - 118
2009 154 5 140
2010 155 22 157
2011 149 33 180
2012 145 44 209
2013 140 57 213
2014 98 98 246
2015 - 120 251
2016 - 127 268
Note: The numbers of domestic and foreign pharmaceutical manufacturers that have passed the
assessments are cumulative every year.
2. Controlled drug licenses and source management
(1) Controlled drugs-related license issuance and training
A. At the end of 2016, 15,413 institutions and companies with controlled drugs
registration licenses, and a total of 52,757 professionals with controlled drugs
prescription licenses had been approved (Refer to Table 4 in Annex II for detailed
license statistics).
B. TFDA issued 95 “import permits”, 650 “permits for importing controlled drugs”,
198 “permits for exporting controlled drugs”, 435 “permits for manufacturing
controlled drugs”, 42 “controlled drugs transportation licenses”, 416 “approval
letter for medical/pharmaceutical education/research trial use”, a total of 1,836
copies in 2016.
C. To improve familiarity with laws and management practices related to controlled
drugs amongst local health bureaus and related businesses, a total of 11 training
courses of controlled drugs management laws were held in 2016.
43