Page 44 - Taiwan Food and Drug Administration 2016 Annual Report
P. 44
Taiwan Food and Drug Adminstration
Policies and Outcomes
1. Amendments to the Schedules of Controlled Drugs
Controlled drugs are divided into four Schedules according to their addictiveness, dependence,
potential for misuse, and social hazards. These Schedules are reviewed by the Ministry of
Health and Welfare Controlled Drugs Review Committee and submitted to the Executive Yuan to
be publicly announced accordingly. The Committee held two meetings in 2015 and added five
additional controlled drugs (listed in Table 2-3-1) in the following Executive Yuan announcement.
Table 2-3-2 lists the number of controlled drugs as of the end of 2015.
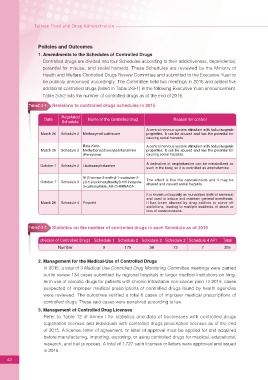
Table2-3-1 Revisions to controlled drugs schedules in 2015
Regulated
Date Name of the controlled drug Reason for control
Schedule
A central nervous system stimulant with hallucinogenic
March 26 Schedule 2 Methoxymethcathinone properties. It can be abused and has the potential for
causing social hazards.
Beta-Keto- A central nervous system stimulant with hallucinogenic
March 26 Schedule 2 Methylbenzodioxolylpentanamine properties. It can be abused and has the potential for
(Pentylone) causing social hazards.
A derivative of amphetamine can be metabolized as
October 7 Schedule 2 Lisdexamphetamine
such in the body, so it is controlled as amphetamine.
N-(1-amino-3-methyl-1-oxobutan-2-
The effect is like the cannabinoids and it may be
October 7 Schedule 3 yl)-1-(cyclohexylmethyl)-1H-indazole-
abused and caused social hazards.
3-carboxamide, AB-CHMINACA
It is known colloquially as niunaizhen (milk of amnesia)
and used to induce and maintain general anesthesia.
March 26 Schedule 4 Propofol It has been abused by drug addicts to stave off
addictions, leading to multiple incidents of death or
loss of consciousness.
表 2-3-2
Table2-3-2 Statistics on the number of controlled drugs in each Schedule as of 2015
chedule of Controlled Drugs Schedule 1 Schedule 2 Schedule 2 Schedule 2 Schedule 4 API Total
Number 9 179 38 73 7 306
2. Management for the Medical-Use of Controlled Drugs
In 2015, a total of 3 Medical-Use Controlled Drug Monitoring Committee meetings were carried
out to review 134 cases submitted by regional hospitals or larger medical institutions on long-
term use of narcotic drugs for patients with chronic intractable non-cancer pain. In 2015, cases
suspected of improper medical prescriptions of controlled drugs found by health agencies
were reviewed. The outcomes verified a total 6 cases of improper medical prescriptions of
controlled drugs. These said cases were penalized according to law.
3. Management of Controlled Drug Licenses
Refer to Table 12 of Annex I for statistics and data of businesses with controlled drugs
registration licenses and individuals with controlled drugs prescription licenses as of the end
of 2015. A license, letter of agreement, or letter of approval must be applied for and acquired
before manufacturing, importing, exporting, or using controlled drugs for medical, educational,
research, and trail purposes. A total of 1,727 such licenses or letters were approved and issued
in 2015.
42