
48
2014 Annual Report
3. Registration Management of Medical Device Manufacturing Factories
Medical device importers may apply for registration letters proving the compliance
of overseas manufacturing factories with the regulation of R.O.C. Quality System
D o c ume n t a t i o n ( QS D ) . T h e
audit inspections for domestic
ma n u f a c t u r e r s a r e p r i ma r i l y
conducted on-site. Importers may
apply for on-site inspections of
overseas manufacturing factories.
By the end of 2013, there were
568 valid registration letters for
domestic medical device GMP, and
3,231registration letters for imported
medical device QSD (Table 4-2).
Section 3 Post-Market Quality Surveillance of Medical Devices
Status
For post-market quality and performance surveillance of medical devices, specific
items were chosen on the basis of risk factor evaluation each year. TFDA integrated the
resources from the local health bureaus to implement the surveillance program in order to
monitor the quality and performance of post- market medical devices.
Policy and Outcome
1. Post-Market Quality and Performance Surveillance of Medical Devices
(1) Risk factor evaluation for the medical devices under surveillance
The surveillance items are chosen based on the risk factors such as medical device
post-market adverse event reports and surveillance reports for defective items. National
samplings are performed for the specific items. The test results provide a complete
quality assessment of the products and prevent possible harm caused by poor quality
of medical devices, and can be used as reference for future policy making on product
quality management.
(2) Results for the post-market quality and performance surveillance
In 2013, a total of 199 samples were inspected for quality performance and package
labeling. There were 174 samples passing the quality performance qualification and the
qualification rate was 93.5% .There were 191 samples passing the package labeling
qualification and the qualification rate was 96%. The devices which failed the inspection
were sent to the local health bureaus to deal with in accordance with the law. The
results of the surveillance are shown in Table 4-3.
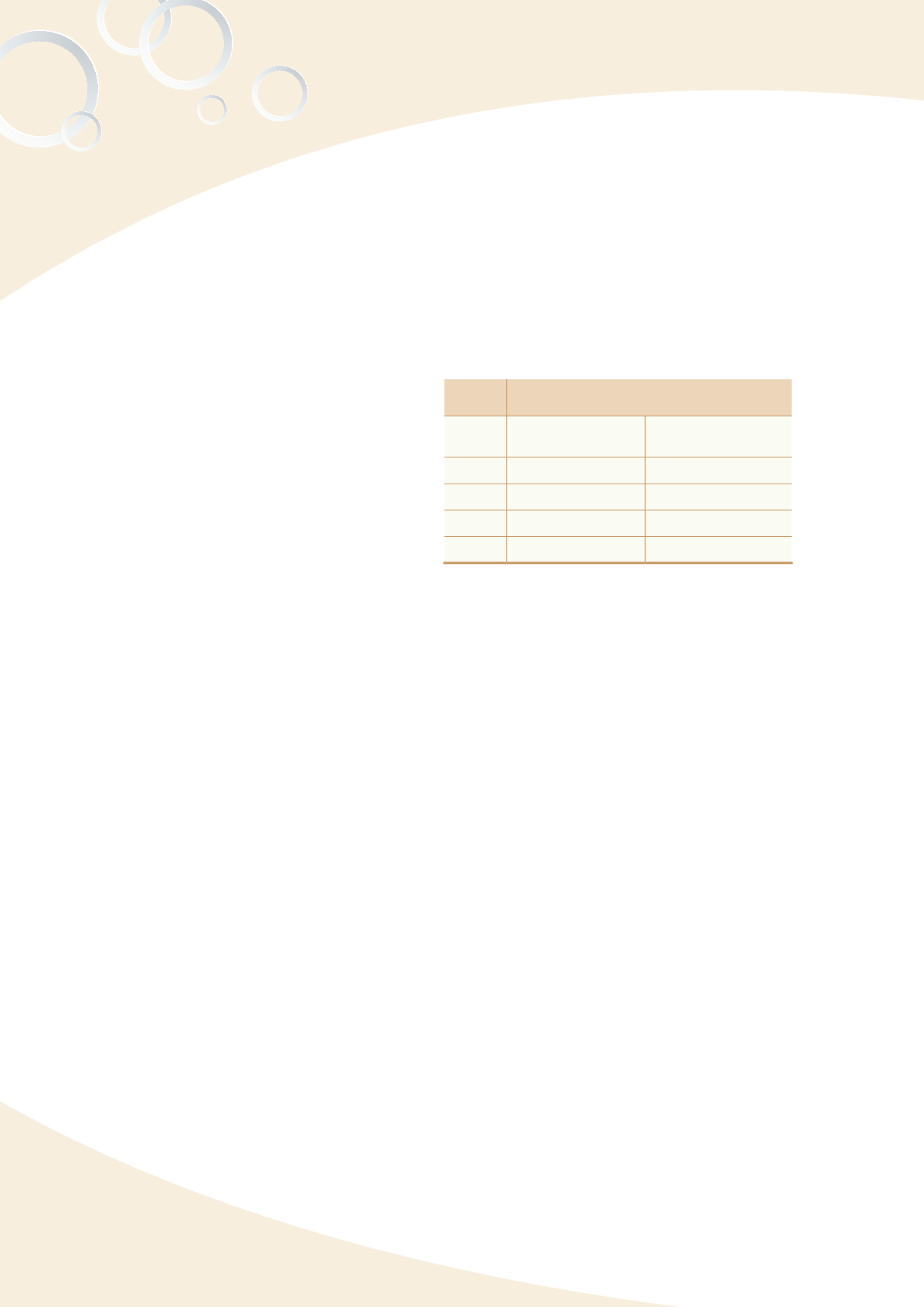
Table 4-2 Number of valid registrations for
medical device GMP/QSD
Valid registration letters for medical device
GMP/QSD
Year
Valid GMP
registration letters
Valid QSD
registration letters
2010
236
1,340
2011
486
2,777
2012
531
3,065
2013
568
3,213



















