
43
Food and Drug Administration
Chapter
︱
Medical Devices Management
︱
4
4LKPJHS KL]PJL PUK\Z[Y` PZ HU LTLYNPUN PUK\Z[Y` [OH[ JHYYPLZ SV[Z VM WV[LU[PHS [V IL
developed in a broad array of dynamic and versatile medical fields. Facing with the
vividness and quality management of domestic medical device industry and the core
emphasis on consumer protection, a total product life cycle regulatory system (Fig. 4-1)
covering international regulatory harmonization, production quality system control, pre-
market control, post-market control, supply chain control, and professional counseling
service was established to effectively ensure the safety, effectiveness, and quality of
medical devices.
Section 1 Medical Device Regulations and Product Review
Status
For marketing application cases of Taiwan-developed innovative products, TFDA
provides regulatory advices and counseling services. These services provide important
niches to assist industrial development.
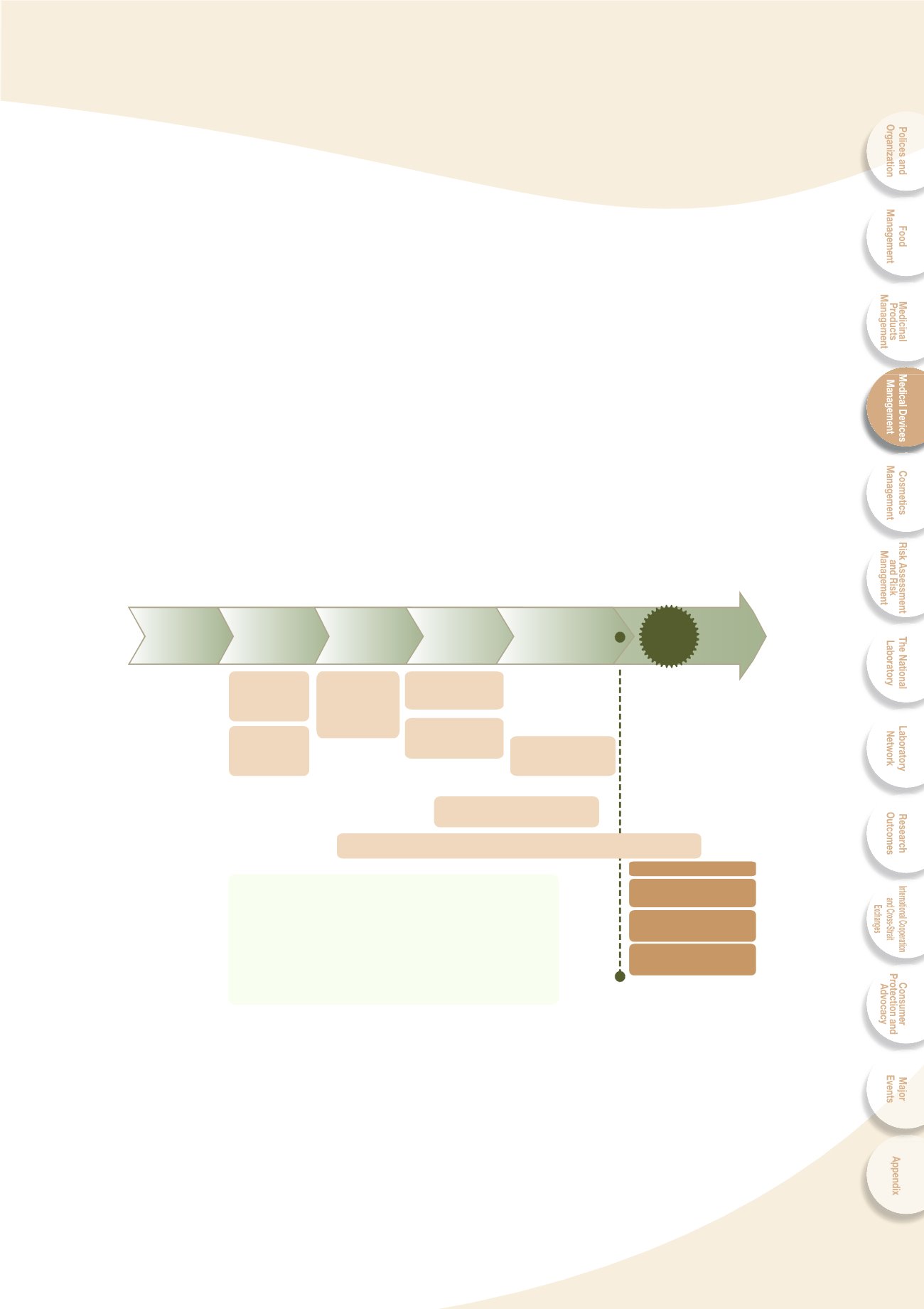
Fig. 4-1 Medical device total product life cycle regulation
ADR & Product
Defect Reporting
GDP
Cosumer Health
Education Prmotion
Safety Surveillance &
Alert Collection (GVP)
MDs
:
Medical Devices
GLP
:
Good Laboratory Practice
GTP
:
Good Tissue Practice
GCP
:
Good Clinical Practice
I R B
:
Institutional Review Board
GMP
:
Good Manufacturing Practice
GDP
:
Good Distribution Practice
ADR
:
Adverse Drug/Device Reaction
GVP
:
Good Vigilance Practice
Medical Care
Needs/
Fundamental
Study
Product
Design/
Prototype
Development
Preclinical
Validation
General/Case
Consultation
Clinical Trial
Inspection (GCP)
Premarket
Control
Postmarket Control
Registration
(Approval/
Listing)
MD Advisory Committee
0DQXIDFWXUHU·V 4XDOLW\ 6\VWHP $XGLW *03
3URGXFWLRQ 4XDOLW\
System Control
Clinical Trial
Protocol Review
(TFDA/IRB)
Product
Designation
Laboratory
Practices
(GLP/GTP)
Clinical Trial
Premarket
Application
Postmarket
Surveillance
Production



















