
38
2014 Annual Report
(2) Constant monitoring illegal food and drugs in market
To stop sales of illegal food and drugs in market, TFDA enhances upstream raw
material and source management by the integration of the government authorities,
and also increases inspection numbers for downstream stores. In addition, TFDA
emphasized education programs through all the media. To prevent illegal food and
drugs advertisements spreading, TFDA and Department of Chinese Medicine and
Pharmacy monitored and reported all regarding to the food, drugs, medical devices
and cosmetic violation advertisement. Once there is a violation, the case will be
transferred to the local health bureau and referee to the local court district attorney. The
results are as follows:
a. In 2013, there were 881 violation cases, while 844 cases were sent to legal agents.
These violations had a total fine of NT$645,000. The local health bureaus had
provided consulting education program to validate the importance.
b. In 2013, there were 6,815 cases and a total fine of NT$152,656,000 involved the
advertisements violation by integration of government authorities, simplification of
inspection process, and the regulation reinforcement.
Section 4 Pharmacovigilance
Status
In recent years, numbers of drugs have been discovered for having unknown or
unexpected side effects (risks) after marketed. In order to enhance patient safety, Taiwan
Food and Drug Administration keep improving drug safety monitoring mechanisms and
regulatory environment (Fig. 3-6).
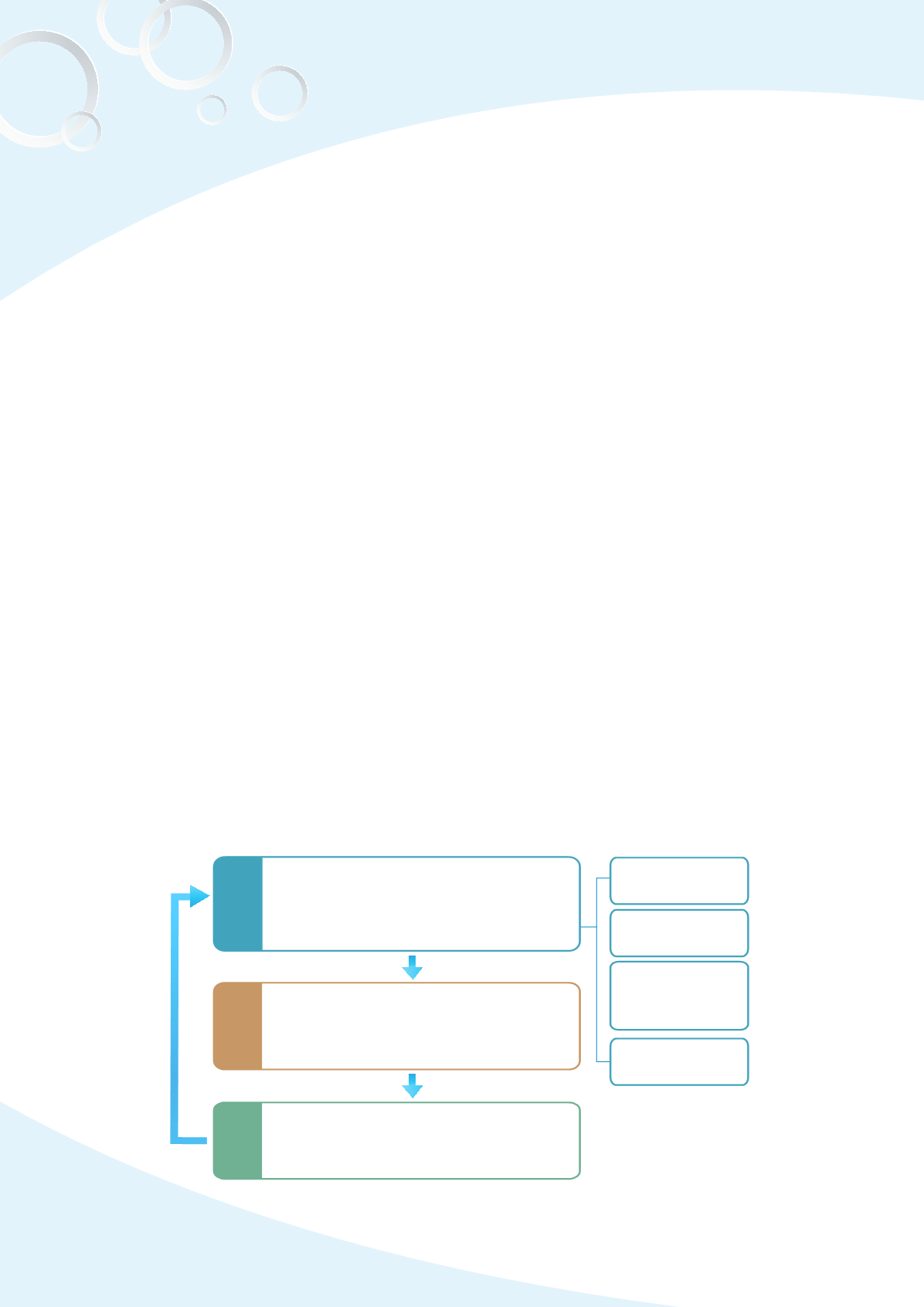
Fig. 3-6 Post-marketing safety surveillance
Problem
Finding
Problem
Analysis
Problem
Solution
Safety monitoring
Collection of drug safety information and
detection of drug potential risk
(e.g. Spontaneous reporting system)
ADR Reporting
system
PSUR
Drug safety news
monitoring
Drug safety
active assess-
ment system
Refinement and assessment
Potential signal refinement and the
benefit/risk of drug assessment
Risk management
Take suitable risk management actions to
minimize the risk (e.g. revision of package
insert, restriction of clinical use, out of sale…)



















