Page 94 - 2021 Taiwan Food and Drug Administration Annual Report
P. 94
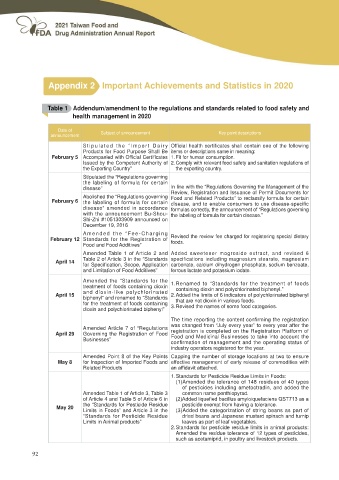
Appendix 2 Important Achievements and Statistics in 2020
Table 1 Addendum/amendment to the regulations and standards related to food safety and
health management in 2020
Date of Subject of announcement Key point descriptions
announcement
S t i p u l a t e d t h e “ I m p o r t D a i r y Official health certificates shall contain one of the following
Products for Food Purpose Shall Be items or descriptions same in meaning:
February 5 Accompanied with Official Certificates 1. Fit for human consumption.
Issued by the Competent Authority of 2. Comply with relevant food safety and sanitation regulations of
the Exporting Country” the exporting country.
Stipulated the “Regulations governing
the labeling of formula for certain In line with the “Regulations Governing the Management of the
disease” Review, Registration and Issuance of Permit Documents for
February 6 Abolished the “Regulations governing Food and Related Products” to reclassify formula for certain
the labeling of formula for certain disease, and to enable consumers to use disease-specific
disease” amended in accordance formulas correctly, the announcement of “Regulations governing
with the announcement Bu-Shou- the labeling of formula for certain disease.”
Shi-Zhi #1051303909 announced on
December 19, 2016
February 12 Amended the “Fee-Charging Revised the review fee charged for registering special dietary
Standards for the Registration of foods.
Food and Food Additives”
Amended Table 1 of Article 2 and A d d e d s w e e t e n e r m o g r o s i d e e x t r a c t , a n d r e v i s e d 6
April 14 Table 2 of Article 3 in the “Standards specifications including magnesium stearate, magnesium
for Specification, Scope, Application carbonate, calcium dihydrogen phosphate, sodium benzoate,
and Limitation of Food Additives” ferrous lactate and potassium iodate.
April 15 Amended the “Standards for the 1. Renamed to “Standards for the treatment of foods
treatment of foods containing dioxin containing dioxin and polychlorinated biphenyl.”
and dioxin-like polychlorinated
biphenyl” and renamed to “Standards 2. Added the limits of 6 indicators of polychlorinated biphenyl
for the treatment of foods containing that are not dioxin in various foods.
dioxin and polychlorinated biphenyl”
3. Revised the names of some food categories.
The time reporting the content confirming the registration
April 29 Amended Article 7 of “Regulations was changed from “July every year” to every year after the
May 8 Governing the Registration of Food registration is completed on the Registration Platform of
Businesses” Food and Medicinal Businesses to take into account the
May 20 confirmation of management and the operating status of
industry operators registered for the year.
Amended Point 8 of the Key Points Capping the number of storage locations at two to ensure
for Inspection of Imported Foods and HႇHFWLYH PDQDJHPHQW RI HDUO\ UHOHDVH RI FRPPRGLWLHV ZLWK
Related Products DQ DႈGDYLW DWWDFKHG
1. Standards for Pesticide Residue Limits in Foods:
(1)Amended the tolerance of 148 residues of 40 types
of pesticides including ametoctradin, and added the
Amended Table 1 of Article 3, Table 3 common name penthiopyrad.
of Article 4 and Table 5 of Article 6 in $GGHG OLTXH¿HG EDFLOOXV DP\ORLTXHIDFLHQV 467 DV D
the “Standards for Pesticide Residue pesticide exempt from having a tolerance.
Limits in Foods” and Article 3 in the (3)Added the categorization of string beans as part of
“Standards for Pesticide Residue dried beans and Japanese mustard spinach and turnip
Limits in Animal products” leaves as part of leaf vegetables.
2. Standards for pesticide residue limits in animal products:
Amended the residue tolerance of 12 types of pesticides,
such as acetamiprid, in poultry and livestock products.
92