Page 40 - 2021 Taiwan Food and Drug Administration Annual Report
P. 40
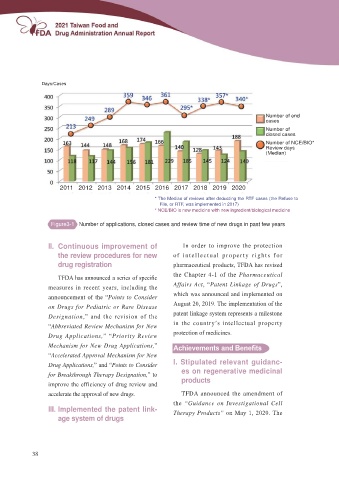
Days/Cases
Number of end
cases
Number of
closed cases
Number of NCE/BIO*
Review days
(Median)
2011 2012 2013 2014 2015 2016 2017 2018 2019 2020
* The Median of reviews after deducting the RTF cases (the Refuse to
File, or RTF, was implemented in 2017)
* NCE/BIO is new medicine with new ingredient/biological medicine
Figure3-1 Number of applications, closed cases and review time of new drugs in past few years
II. Continuous improvement of In order to improve the protection
the review procedures for new of intellectual property rights for
drug registration SKDUPDFHXWLFDO SURGXFWV 7)'$ KDV UHYLVHG
the Chapter 4-1 of the Pharmaceutical
7)'$ KDV DQQRXQFHG D VHULHV RI VSHFL¿F Affairs Act ³Patent Linkage of Drugs´
PHDVXUHV LQ UHFHQW \HDUV LQFOXGLQJ WKH which was announced and implemented on
announcement of the “Points to Consider $XJXVW 7KH LPSOHPHQWDWLRQ RI WKH
on Drugs for Pediatric or Rare Disease patent linkage system represents a milestone
Designation ´ DQG WKH UHYLVLRQ RI WKH in the country’s intellectual property
“Abbreviated Review Mechanism for New protection of medicines.
Drug Applications,´ ³Priority Review
Mechanism for New Drug Applications,´ $FKLHYHPHQWV DQG %HQH¿WV
“Accelerated Approval Mechanism for New
Drug Applications,´ DQG ³Points to Consider I. Stipulated relevant guidanc-
for Breakthrough Therapy Designation,´ WR es on regenerative medicinal
improve the efficiency of drug review and products
accelerate the approval of new drugs.
TFDA announced the amendment of
III. Implemented the patent link- the “Guidance on Investigational Cell
age system of drugs Therapy Products” RQ 0D\ 7KH
38