Page 50 - 2019食藥署年報(英文版)
P. 50
/VNCFS PG /VNCFS PG /VNCFS PG
/VNCFS
EBUF
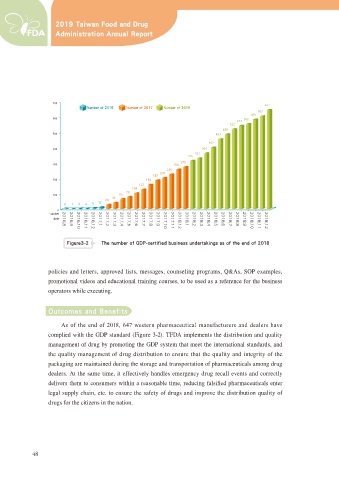
'JHVSF cc 5IF OVNCFS PG (%1 DFSUJGJFE CVTJOFTT VOEFSUBLJOHT BT PG UIF FOE PG
policies and letters, approved lists, messages, counseling programs, Q&As, SOP examples,
promotional videos and educational training courses, to be used as a reference for the business
operators while executing.
0VUDPNFT BOE #FOFGJUTVUDPNFT BOE #FOFGJUT
0
$V RI WKH HQG RI ZHVWHUQ SKDUPDFHXWLFDO PDQXIDFWXUHUV DQG GHDOHUV KDYH
complied with the GDP standard (Figure 3-2). TFDA implements the distribution and quality
management of drug by promoting the GDP system that meet the international standards, and
the quality management of drug distribution to ensure that the quality and integrity of the
packaging are maintained during the storage and transportation of pharmaceuticals among drug
dealers. At the same time, it effectively handles emergency drug recall events and correctly
GHOLYHUV WKHP WR FRQVXPHUV ZLWKLQ D UHDVRQDEOH WLPH UHGXFLQJ IDOVL¿HG SKDUPDFHXWLFDOV HQWHU
legal supply chain, etc. to ensure the safety of drugs and improve the distribution quality of
drugs for the citizens in the nation.
48