Page 45 - 2019食藥署年報(英文版)
P. 45
match the purchased data, after being judged to be in compliance with the regulations, it can be
released.
0VUDPNFT BOE #FOFGJUTVUDPNFT BOE #FOFGJUT
0
1SPNPUJOH UIF MFHJTMBUJPO PG UIF TQFDJBM MBX GPS UIF NBOBHFNFOU PG
SFHFOFSBUJWF NFEJDJOBM QSPEVDUT
&RQVLGHULQJ WKH KHWHURJHQHLW\ RI LQJUHGLHQWV SURFHVV VSHFL¿FLW\ DQG WUHDWPHQW FRPSOH[LW\
of regenerative medicinal products, the current regulations cannot be fully applied. Through
the promotion of the special law of the Pharmaceutical Affairs Act, it is expected to help to
IDFLOLWDWH HIIHFWLYH FRPPXQLFDWLRQ DQG UHLQIRUFH WKH DUHDV WKDW DUH QRW VSHFL¿FDOO\ FRYHUHG LQ WKH
Pharmaceutical Affairs Act, in order to improve the regulatory environment for the total product
life cycle management of regenerative medicinal products. At present, Regenerative Medicinal
Products Derivative Act (Draft) has been reviewed by the Legislative Yuan. We look forward
to the early completion of legislation to provide more and more innovative treatment options for
patients and to promote the vigorous development of Taiwan’s biotechnology industry chain.
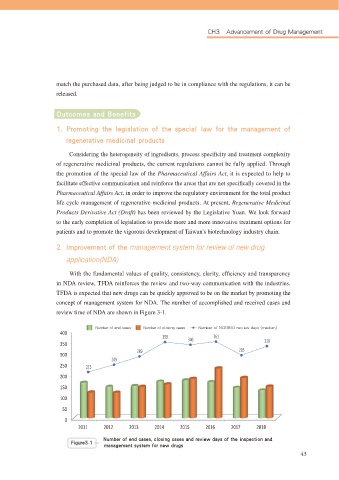
*NQSPWFNFOU PG UIF management system for review of new drug
application(NDA)
:LWK WKH IXQGDPHQWDO YDOXHV RI TXDOLW\ FRQVLVWHQF\ FODULW\ HI¿FLHQF\ DQG WUDQVSDUHQF\
in NDA review, TFDA reinforces the review and two-way communication with the industries.
TFDA is expected that new drugs can be quickly approved to be on the market by promoting the
concept of management system for NDA. The number of accomplished and received cases and
review time of NDA are shown in Figure 3-1.
/VNCFS PG FOE DBTFT /VNCFS PG DMPTJOH DBTFT /VNCFS PG /$& #*0 SFWJFX EBZT NFEJBO
400
359 346 361
350 339
289 295
300
249
250 213
200
150
100
50
0
2011 2012 2013 2014 2015 2016 2017 2018
cc/VNCFS PG FOE DBTFT DMPTJOH DBTFT BOE SFWJFX EBZT PG UIF JOTQFDUJPO BOE
'JHVSF
NBOBHFNFOU TZTUFN GPS OFX ESVHT
43